Article Text
Statistics from Altmetric.com
Overview
An ectopic pregnancy occurs when a fertilised ovum implants outside the normal uterine cavity.1,–,3 It is a common cause of morbidity and occasionally of mortality in women of reproductive age. The aetiology of ectopic pregnancy remains uncertain although a number of risk factors have been identified.4 Its diagnosis can be difficult. In current practice, in developed countries, diagnosis relies on a combination of ultrasound scanning and serial serum beta-human chorionic gonadotrophin (β-hCG) measurements.5 Ectopic pregnancy is one of the few medical conditions that can be managed expectantly, medically or surgically.1 3 6
Incidence
In the developed world, between 1% and 2% of all reported pregnancies are ectopic pregnancies (comparable to the incidence of spontaneous twin pregnancy).7 The incidence is thought to be higher in developing countries, but specific numbers are unknown. Although the incidence in the developed world has remained relatively static in recent years, between 1972 and 1992 there was an estimated six-fold rise in the incidence of ectopic pregnancy.8 This increase was attributed to three factors: an increase in risk factors such as pelvic inflammatory disease and smoking in women of reproductive age, the increased use of assisted reproductive technology (ART) and increased awareness of the condition, facilitated by the development of specialised early pregnancy units (EPUs).
Morbidity and mortality
In the UK, ectopic pregnancy remains the leading cause of pregnancy-related first trimester death (0.35/1000 ectopic pregnancies).3 6 9 However, in the developing world it has been estimated that 10% of women admitted to hospital with a diagnosis of ectopic pregnancy ultimately die from the condition.10 Ectopic pregnancy is a considerable cause of maternal morbidity, causing acute symptoms such as pelvic pain and vaginal bleeding and long-term problems such as infertility.3 Short- and long-term consequences of ectopic pregnancy on health-related quality of life and on bereavement issues are likely to be significant but have not been formally quantified.
▶ Clinicians should be suspicious of ectopic pregnancy in any woman of reproductive age presenting with abdominal or pelvic symptoms.
▶ The diagnosis of ectopic pregnancy can be difficult and protracted.
▶ A diagnosis of ‘pregnancy of unknown location’ should trigger further diagnostic pathways and follow-up until the final outcome of the pregnancy is known.
▶ Medical management with methotrexate is successful for small, stable ectopic pregnancies.
Risk factors
Although women with ectopic pregnancy frequently have no identifiable risk factors, a prospective case-controlled study has shown that increased awareness of ectopic pregnancy and a knowledge of the associated risk factors helps identify women at higher risk in order to facilitate early and more accurate diagnosis.11 Most risk factors are associated with risks of prior damage to the Fallopian tube (Box 1). These factors include any previous pelvic or abdominal surgery, and pelvic infection.11 Chlamydia trachomatis has been linked to 30–50% of all ectopic pregnancies.12 The exact mechanism of this association is not known but it has been proposed that in addition to distortion of tubal architecture, it may to be due to an effect on the tubal microenvironment.13
Risk factors for ectopic pregnancy
▶ Fallopian tube damage
Previous tubal surgery (including female sterilisation) and pelvic surgery including Caesarean section and ovarian cystectomy
Previous abdominal surgery including appendicectomy and bowel surgery
Confirmed genital infection and pelvic inflammatory disease, commonly caused by chlamydial infection
▶ Infertility
Documented tubal disease
Assisted reproductive technology
Endometriosis
Unexplained infertility
▶ Contraceptive failure
Progestogen-only contraception
Intrauterine contraceptive device
▶ Cigarette smoking – including past exposure.
▶ Age >35 years
▶ Previous ectopic pregnancy
▶ Previous spontaneous abortion or induced abortion
Ectopic pregnancy is more common in women attending infertility clinics14 even in the absence of tubal disease. In addition, the use of ART increases the rate of ectopic pregnancies. In vitro fertilisation (IVF) is associated with an ectopic pregnancy risk of 2–5% and it may be higher than this where there is tubal disease. Indeed the first IVF pregnancy, before the first IVF live birth, was a tubal ectopic pregnancy.15
Some types of contraception, such as progestogen-only contraception and the intrauterine contraceptive device, are associated with an increased incidence of ectopic pregnancy when there is contraceptive failure, without necessarily increasing the absolute risk of ectopic pregnancy.16
One third of all cases of ectopic pregnancy are thought to be associated with smoking.17 There is a dose–effect relationship, with the highest adjusted odds ratio (OR) (3.9) when more than 20 cigarettes are smoked a day.18 Several mechanisms for this association have been suggested, including one or more of the following: delayed ovulation, altered tubal and uterine motility and microenvironment, or altered immunity.19 20
The risk of ectopic pregnancy increases with advancing maternal age, with age over 35 years being a significant risk factor.6 Hypotheses for this association include the higher probability of exposure to most other risk factors with advancing age, increase in chromosomal abnormalities in trophoblastic tissue and age-related changes in tubal function delaying ovum transport, resulting in tubal implantation.18
Women with a previous history of ectopic pregnancy also have an increased risk, which increases further in proportion to the number of previous ectopic pregnancies. In one study the OR for having an ectopic pregnancy was 12.5 after one previous ectopic pregnancy and 76.6 after two.18
Aetiology
The exact aetiology of ectopic pregnancy is unknown. It is notable that it is unique to humans, and perhaps the higher apes, so that there are no good animal models that could be used to further our understanding.21 However, it is thought that tubal implantation occurs as a result of a combination of arrest of the embryo in the Fallopian tube and changes in the tubal microenvironment that allow early implantation to occur.4 Inflammation within the tube, resulting from infection or smoking, may affect embryo-tubal transport by disrupting smooth muscle contractility and ciliary beat activity and may also provide pro-implantation signals. Molecular research generally involves studying Fallopian tube biopsies taken from women with ectopic pregnancies. Interpretation is limited as comparable Fallopian tube samples are not available from women with an intrauterine pregnancy (IUP) or in advance of an ectopic pregnancy occurring. Thus, it is difficult to ascertain whether any molecular changes observed are a cause or a consequence of ectopic implantation. Novel studies focusing on the functional consequences of smoking and infection on Fallopian tube physiology and pathobiology are required.
Clinical presentation
Patients with an ectopic pregnancy commonly present with pain and vaginal bleeding between 6 and 10 weeks' gestation.1 However, these are common symptoms in early pregnancy, with one third of women experiencing some pain and/or bleeding.22,–,24 The pain can be persistent and severe and is often unilateral. However unilateral pain is not always indicative of ectopic pregnancy as, in early pregnancy, a prominent painful ovarian corpus luteum cyst is common. Shoulder tip pain, syncope and shock occur in up to 20% of women and abdominal tenderness in more than 75%. Bimanual examination, if performed at all, should be done cautiously and gently. Cervical motion tenderness has been reported in up to 67% of cases, and a palpable adnexal mass in about 50%.23,–,25 More recently, it has been reported that one third of women with ectopic pregnancy have no clinical signs and 9% have no symptoms.26 27
A ruptured ectopic pregnancy should be strongly suspected if a woman has a positive pregnancy test and presents with syncope and signs of shock including tachycardia, pallor and collapse. There may be abdominal distension and marked tenderness. While a bimanual examination may reveal tenderness, cervical excitation and an adnexal mass, great caution is required as this may exacerbate bleeding. As ectopic pregnancy affects young, fit women they are often able to mount remarkable haemodynamic compensation. Tachycardia is a particularly important sign, but decompensation with shock is a sign of significant intraperitoneal bleeding. In an emergency, where the patient has collapsed and there is high clinical suspicion of tubal rupture, extensive clinical examination is inappropriate and immediate surgical intervention is indicated.
Unfortunately, atypical presentation is also relatively common. Ectopic pregnancy may mimic other gynaecological disorders and gastrointestinal or urinary tract disease, including appendicitis, salpingitis, ruptured corpus luteum or follicular cysts, threatened or inevitable spontaneous abortion, ovarian torsion and urinary tract infection. The 1997–1999 and 2003–2005 Confidential Enquiries into Maternal Deaths reports highlighted that most of the women who died from ectopic pregnancy were misdiagnosed in the primary care or accident and emergency settings.28 29 It was therefore recommended that all clinicians should be made aware of the atypical clinical presentations of ectopic pregnancy. While there has been a welcome decline in the case death rate in women with ectopic pregnancies, a key lesson emphasised in these reports does not appear to have been learnt. In the 2006–2008 Centre for Maternal and Child Enquiries (CMACE) report, four of the six women who died from early ectopic pregnancy complained of diarrhoea, dizziness or vomiting as early symptoms, without triggering any consideration of extrauterine pregnancy by their medical attendants.30
However, it remains difficult to diagnose an ectopic pregnancy from risk factors, history and examination alone. Clinicians should be suspicious of pregnancy in any such woman who presents with abdominal or pelvic symptoms and should always bear in mind the possibility of ectopic pregnancy in any woman of reproductive age who presents with any of the symptoms mentioned above.
Diagnosis
Diagnosis of ectopic pregnancy has improved significantly due to advances in ultrasound technology, rapid and sensitive serum hormone assays, the development of EPUs and an increased awareness and understanding of the associated risk factors. Despite this, around half of the women with an eventual diagnosis of ectopic pregnancy are not diagnosed at their first presentation.31 32 Early diagnosis reduces the risk of tubal rupture and allows more conservative medical treatments to be employed.1 33
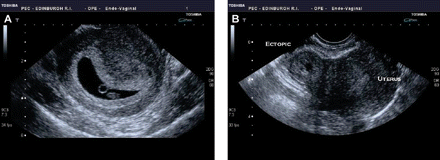
Currently, diagnosis in unruptured ectopic pregnancy is achieved using a combination of transvaginal ultrasonography and measurement of serum β-hCG concentrations. One of the key elements in the diagnosis is the exclusion of a viable or non-viable IUP. Diagnosis can be straightforward when a transvaginal ultrasound scan (TVS) positively identifies an IUP or ectopic pregnancy34 (Figure 1). However, TVS fails to identify the location of a pregnancy in a significant number of women and such women are currently diagnosed as having a ‘pregnancy of unknown location’ (PUL).35 36
Transvaginal ultrasound images of an intrauterine pregnancy (IUP) and ectopic pregnancy. (A) An IUP at 6 weeks. The central dark area is the intrauterine gestational sac and within the sac is a circular ringed structure that is the yolk sac. The small oval structure below the yolk sac is the fetus. (B) An ectopic pregnancy. To the right of the image is the normal uterus and to the left of the uterus is the doughnut-shaped ectopic pregnancy.
The 2006–2008 CMACE report drew attention to a maternal death secondary to ruptured ectopic pregnancy where a diagnosis of PUL had been made.30 Although most patients with a PUL will subsequently be diagnosed with either a failed IUP (a spontaneous abortion) or viable IUP, the report highlights that 7–20% will be diagnosed with an ectopic pregnancy. It is therefore very important that a diagnosis of PUL should trigger further diagnostic pathways and follow-up until the final outcome of the pregnancy is known.
The concept of a ‘discriminatory β-hCG level’ was introduced in 1985 to highlight the serum concentration of β-hCG when a pregnancy should be visible on an ultrasound scan. Using transabdominal ultrasound examination, it was reported then that the absence of an intrauterine gestational sac at a β-hCG concentration over 6500 IU/l had a sensitivity of 100%, specificity of 96%, positive predictive value of 87% and negative predictive value of 100% for the prediction of ectopic pregnancy. In the context of a 19.4% prevalence of ectopic pregnancies in the study group, this diagnostic paradigm was 98% efficient.37 With the introduction of high-resolution TVS, the discriminatory β-hCG level of 6500 IU/l is now less helpful.35 38 An ectopic pregnancy can be detected at β-hCG concentrations well below this level and an ultrasound scan should not be delayed because of low β-hCG concentrations.
Transvaginal ultrasonography
High-definition ultrasonography, particularly using the transvaginal route, has revolutionised the assessment of patients with early pregnancy problems, allowing for clearer visualisation of both normal and abnormal gestations.39 In a healthy IUP, a TVS should identify the intrauterine gestation sac with almost 100% accuracy at a gestational age of 5.5 weeks.40 41 Even so, it is recognised radiographic practice that an IUP is only definitively diagnosed by ultrasound visualisation of a yolk sac or embryo in addition to a gestation sac.42,–,44 This is because an ectopic pregnancy can be accompanied by a ‘pseudosac’, a collection of fluid within the endometrial cavity that may be the result of localised breakdown of the decidualised endometrium. However, its central location within the endometrial cavity distinguishes it from the very early gestation sac that is typically eccentrically placed.45 In addition, pseudosacs are transient rather than consistent and they do not have a hyperechoic decidual reaction around them. Additional embryonic features including the yolk sac and cardiac activity should be clearly visible after 6 weeks' gestation. A sonographer with experience in early pregnancy scanning should generally be able to tell the difference between a pseudosac and an empty early intrauterine sac.
The identification of an IUP can rule out ectopic pregnancy in most settings unless a heterotopic pregnancy is suspected, where an ectopic pregnancy coexists with an IUP.46 They are rare (1 in 40 000), although more common after assisted conception, and difficult to diagnose.
In the absence of an intrauterine gestation sac, an ectopic pregnancy can be diagnosed by the presence of an adnexal mass, often visible within the Fallopian tube. The positive identification of a non-cystic adnexal mass with an empty uterus has a sensitivity of 84–90% and a specificity of 94–99% for the diagnosis of an ectopic gestation.47 In one large prospective study of 6621 patients, ectopic pregnancy was correctly diagnosed by TVS with a sensitivity of 90.9% and specificity of 99.9%.24 False positives can, however, occur if other structures such as the corpus luteum, bowel, a paratubal cyst, a hydrosalpinx or an endometrioma are mistaken for an ectopic pregnancy. False negatives can occur if the ectopic is small or if it is concealed by bowel or uterine anomalies such as fibroids. It is therefore possible for an ectopic pregnancy to go unnoticed on an ultrasound scan, especially if the patient is asymptomatic.
Around 80% of ectopic pregnancies will be on the same side as the ovarian corpus luteum, the identification of which can help in the search for an adnexal mass. The mass may appear as an inhomogenous echogenic area adjacent to the ovary that moves separately from it on gentle pressure; a gestation sac enclosed by a hyperechoic ring (the so-called ‘bagel’ appearance); or a gestation sac with a fetal pole, with or without cardiac activity.
Suspicion of an ectopic pregnancy increases if free fluid (representing blood) is visualised, either surrounding the uterus or in the Pouch of Douglas,48 although a small amount of free fluid in the Pouch of Douglas, a transudate due to increased vascular permeability, is common in early pregnancy.
Box 2 summarises ultrasonographic findings that are useful in diagnosing an ectopic pregnancy.
Useful ultrasonographic findings in the diagnosis of ectopic pregnancy
▶ Absence of intrauterine pregnancy (IUP)
▶ Positive identification of an ectopic pregnancy mass: inhomogenous mass, empty adnexal gestation sac or adnexal sac containing yolk sac or fetal pole
▶ Free fluid (i.e. blood): suggestive of ectopic pregnancy in the absence of IUP, but not diagnostic (small amount may be physiological)
Serum β-hCG concentrations
The changes in serum β-hCG concentrations over time have been used to predict the outcome of PULs.49 Kadar and Romero50 were the first to describe these serial changes on the basis of a small sample of 20 women using an 85% confidence interval (CI). They showed that in a normal ongoing pregnancy, the minimal rate of increase in β-hCG is 66% in 2 days. In a recent study of 287 patients with pain or bleeding, the minimum rise in β-hCG for a viable IUP was 24% at 24 hours and 53% at 48 hours.51 In addition, Seeber et al.52 produced data with a 99% CI that suggested a more conservative minimum rise of 35% over 2 days. In current practice most units use a minimum value of between 50% and 66% for the acceptable 48-hour increase in β-hCG in a normal pregnancy.53 Some non-viable IUPs will also demonstrate an exponential increase in serum β-hCG, so normal β-hCG changes do not necessarily confirm viability. However, absence of this expected rise suggests early pregnancy failure.
A rapid decline in β-hCG concentrations over 2 days, commonly by 21–35% or more, is indicative of a spontaneous abortion52 or a resolving ectopic pregnancy. In an ectopic pregnancy, β-hCG concentrations are just as likely to fall as to rise, with no single pattern able to characterise the condition.54 However, 71% have serial serum β-hCG values that increase more slowly than would be expected with a viable IUP and decrease more slowly than would be expected with a spontaneous abortion.9
If the history is not compatible with a spontaneous abortion, or the β-hCG concentrations continue to rise and the scan location of the pregnancy is still unknown, an ectopic pregnancy is likely and a clear management strategy should be put in place.
Serum progesterone
Although there are no definitive values that demarcate an ectopic pregnancy from an IUP, the measurement of serum progesterone levels is a potentially useful adjunct in the assessment of PULs.55 Serum progesterone concentrations in a viable IUP are >50 ng/ml. Although progesterone assessment cannot easily discriminate between an ectopic pregnancy and a failing IUP56 some EPUs use a low progesterone (<5 ng/ml) to differentiate between ‘low-risk’ patients, when a PUL may be suitable for conservative management, and ‘at-risk’ patients who require definitive treatment.57
Other serum biomarkers
Although other potential serum biomarkers have been proposed,58 none of these are used in common clinical practice. New biomarkers with clinical utility would be helpful in improving the diagnosis of ectopic pregnancy, with the potential benefits of greater safety and reduced diagnostic costs.5 32
Diagnostic laparoscopy
In cases where an ectopic pregnancy is suspected and ultrasound is inconclusive, a diagnostic laparoscopy may be required. This is believed by many to be the ‘gold standard’ investigation in ectopic pregnancy. Indeed reluctance or delay in performing a diagnostic laparoscopy has been highlighted as a factor in fatal cases.30 However, some small ectopic pregnancies may be missed at the time of laparoscopy or laparotomy. In one study, 2 of 44 (4.5%) women reported to have no evidence of an ectopic pregnancy at the time of laparoscopy were subsequently diagnosed with one.55 An alternative to diagnostic laparoscopy may involve a repeat ultrasound examination, particularly when β-hCG concentrations are close to 1500 IU/l. Other strategies include alternative diagnostic tests, such as serum progesterone or an endometrial biopsy, or empirical medical treatment as the patient may well have an ectopic pregnancy. If β-hCG concentrations are falling but an ectopic has not been excluded, consideration should be given to performing serial β-hCG measurements until levels become undetectable, as rupture can still occur.40
Endometrial biopsy
In selected cases of PUL, an endometrial biopsy may be taken and analysed for the presence or absence of chorionic villi. Their absence in the presence of a static β-hCG is suggestive of an ectopic pregnancy. A dilatation and curettage may be useful when performed in association with a ‘negative’ diagnostic laparoscopy for a suspected ectopic pregnancy. The clinician should be certain that the pregnancy, if intrauterine, is non-viable and appropriate consent obtained, as this procedure could potentially interrupt a continuing pregnancy.
Management
Ectopic pregnancy may be managed surgically, medically or expectantly. In these days of increasing outpatient diagnosis and management it is important to remember the risks of ruptured ectopic pregnancy. Clear documentation of diagnostic and management strategies – with clinical, sonographic and biochemical assessment of the patient – is therefore important. Which management is most appropriate depends on ongoing assessment and on numerous clinical factors. Management is tailored to individual patients, based on their presentation and on the severity of their condition, suitability of treatment options and patient preference. Figure 2 demonstrates a suggested diagnosis and management pathway.
Recommended diagnostic and management approach for suspected ectopic pregnancy. It is important to highlight that the figure of 66% is used as a practical guide only and that all cases of pregnancy of unknown location should be considered as a potential ectopic pregnancy until assessment proves otherwise or management is complete. β-hCG, beta-human chorionic gonadotrophin.
Surgery
Surgical management is imperative in the clinical scenario of a ruptured ectopic pregnancy. A laparoscopic approach is preferable to an open approach in a patient who is haemodynamically stable. Laparoscopic procedures are associated with shorter operative times, less intraoperative blood loss, shorter hospital stays and lower analgesia requirements.59,–,61 Laparotomy should be reserved for patients who present with rupture and are in a state of hypovolaemic shock and compromise. If the contralateral tube is healthy, the preferred option is salpingectomy, where the entire Fallopian tube, or the affected segment containing the ectopic gestation, is removed (Figure 3). A salpingostomy is the removal of the ectopic pregnancy, by dissecting it out of the tube, leaving the Fallopian tube in situ in an attempt to preserve fertility on that side.
(A) Left tubal ectopic pregnancy at laparoscopy. (B) Tubal ectopic pregnancy has been removed by salpingectomy.
A number of systematic reviews have examined reproductive outcomes following the two procedures in patients with a healthy contralateral tube. Studies in this area can be criticised with regard to patient selection, surgical techniques and follow-up times62,–,64 and some studies report conflicting results.65 66 However, it is generally accepted that the chance of subsequent IUP is not increased after salpingostomy compared with salpingectomy. In addition, the use of conservative surgical techniques exposes women to a small risk of tubal bleeding in the immediate postoperative period and the potential need for further treatment of persistent trophoblast.9 This supports current guidelines stating that the operation of choice, where there is a healthy contralateral tube, is laparoscopic salpingectomy.67
In the presence of contralateral tubal disease, a laparoscopic salpingostomy should be considered if future fertility is desired. Persistent trophoblast is the main concern after a salpingostomy. This is usually detected by a failure of serum β-hCG levels to fall and is more common following active tubal bleeding, where the ectopic pregnancy size was >2 cm or if serum β-hCG concentrations are >3000 IU/l or rising prior to surgery.68 Women should be followed up with serial β-hCG measurements and systemic methotrexate treatment may be required if the levels fail to fall as expected. While the short-term costs of postoperative follow-up and treatment of persistent trophoblast are greater following a salpingostomy,69 the potential avoidance of the subsequent need for assisted conception will make it more cost effective compared with salpingectomy.66
Medical treatment with methotrexate
Medical treatment is useful for patients with an unruptured tubal ectopic pregnancy who are haemodynamically stable and have minimal symptoms and a low volume of free intraperitoneal fluid on ultrasound scan.70 Intramuscular methotrexate is the most widely used and successful medical therapy for ectopic pregnancy and is generally administered in a single-dose protocol.34 69 Methotrexate is a folic acid antagonist that targets rapidly dividing cells and arrests mitosis.9 71 In ectopic pregnancy, the drug prevents the proliferation of cytotrophoblast cells, reducing cell viability and β-hCG secretion and thus progesterone support for the pregnancy. This facilitates the resolution of the ectopic pregnancy and tissue remodelling.
After assessing patient suitability for medical management (Box 3), body surface area is calculated using height and weight measurements. In addition, a baseline full blood count and renal and liver function tests are obtained. In general, apart from some abdominal discomfort 1–3 days after treatment and abdominal bloating, side effects are not common and return to normal activities is quicker than after surgery. Potential serious side effects such as significant hepatotoxicity, bone marrow toxicity or alopecia are extremely rare with ectopic pregnancy treatment regimens. Patients require careful monitoring to ensure complete resolution of the ectopic gestation using serial assessment of β-hCG levels every 4–7 days (protocols vary between units) until the β-hCG level is <5 IU/l.72
Inclusion criteria for medical management of ectopic pregnancy with methotrexate
▶ Patient characteristics
Would prefer medical option
Willing to attend follow-up for up to 6 weeks
Willing to abstain from alcohol for 7 days following the treatment
Not breastfeeding or willing to stop
▶ Clinical features
Haemodynamically stable
Minimal abdominal pain
▶ Ultrasound scan findings
No fetal heart activity or clear yolk sac in adnexal mass
Small amount of free fluid
Unlikely to be early intrauterine pregnancy failure
▶ Serum beta-human chorionic gonadotrophin (β-hCG) concentrations
Usually <3000 IU/l (Although limits of <5000 IU/l are used in some units and earlier studies, treatment success rates are higher when this more commonly used lower limit applies.)
▶ Medical history
No active peptic ulcer disease
No severe medical conditions including renal disease, hepatic disease, severe anaemia, leucopenia or thrombocytopenia
▶ Should not be on concurrent medication
Non-steroidal anti-inflammatory agents (NSAIDs), aspirin, penicillins, sulphonamides, trimethoprim, tetracyclines, diuretics, phenytoin, antimalarials, ciclosporin, retinoids, probenecid, folic acid, hypoglycaemics, live vaccines, nephrotoxic or hepatotoxic drugs
The commonly used single-dose methotrexate treatment regimen involves a deep intramuscular injection at a dose of 50 mg/m2 of the calculated body surface area. Approximately 14–20% of patients receiving single-dose treatment will require a repeat dose,73 74 usually decided on following a fall of the β-hCG concentration of less than 15% from Day 4 to 7 after treatment. This timescale is used as methotrexate can cause a transient rise in serum β-hCG after initial treatment. Approximately 10% of women will require surgical intervention,75 although most of these are for slowly falling β-hCG levels rather than for acute tubal rupture. However, rupture still remains a possibility during treatment. Close treatment surveillance, and staff and patient awareness of potential treatment failure, are vital.
Two much less common uses of methotrexate for the treatment of ectopic gestation are the multi-dose protocol and direct injection of methotrexate into the ectopic pregnancy. The multi-dose regimen consists of methotrexate treatment on Days 1, 3, 5 and 7 to a maximum of four doses and leucovorine ‘rescue-therapy’ at a dose of 0.1 mg/kg on alternate Days 2, 4, 6 and 8. This treatment may be more appropriate for patients who present with a larger adnexal masses and greater initial β-hCG levels (>5000 IU/l). Direct injection of methotrexate into the ectopic sac, either laparoscopically or with ultrasound guidance, limits systemic toxicity and maintains a higher therapeutic level. However, local injection has no significant advantage in most patients and is accompanied by a risk of provoking tubal rupture.
Methotrexate treatment is very successful for small stable ectopic pregnancies. A meta-analysis of non-randomised studies showed success rates of 93% (95% CI 89–96%) for multi-dose protocols and 88% (95% CI 86–90%) for single dose therapy.76 Failure of single-dose medical management is associated with initial serum β-hCG concentrations >5000 IU/l, a moderate or large amount of free fluid on ultrasound, the presence of fetal cardiac activity and a pretreatment increase in serum β-hCG of >50% over a 48-hour period. It is not known whether methotrexate treatment has better fertility outcomes than surgery but this is likely to be the case when the ectopic gestation occurs in the only functioning tube.
Expectant management
Some ectopic pregnancies resolve spontaneously through either regression or tubal abortion, without causing harm to the patient. Expectant management is a conservative strategy consisting of observation and assessment of whether the ectopic pregnancy is continuing to resolve spontaneously and successfully without intervention.34 A suitable candidate for expectant management must have an ectopic pregnancy with no evidence of rupture, be clinically stable and asymptomatic, and have consistently declining β-hCG concentrations. A low serum progesterone is also a possible marker of suitability for the expectant approach. Follow-up should be between one and three times weekly with β-hCG measurement and ultrasonography as required. Expectant management is reported to be most useful when the initial β-hCG is <1000 IU/l.58 A rapidly declining β-hCG level also appears to predict a favourable outcome.77 Success rates between 47% and 82% are reported, depending on the patient's initial status.78
The importance of compliance with follow-up and ease of access to the hospital should be emphasised. If β-hCG levels remain static or decline suboptimally, consideration should be given to reverting to surgical or medical management.
Unusual sites of implantation
Over 98% of ectopic pregnancies implant in the Fallopian tube, in its ampullary region (70%), isthmus (12%) or fimbria (11.1%). Interstitial or cornual ectopics, where the pregnancy implants in the intramyometrial portion of the Fallopian tube, are less common (2.4%) but have a mortality twice that of any other type of Fallopian tube ectopic pregnancy.77 Rarely, an ectopic pregnancy implants at an extratubal location, such as the cervix, ovary, abdomen, liver, spleen or Caesarean section scar.1 This produces a diagnostic challenge and colour Doppler visualisation aids in the identification of the ectopic pregnancy by creating awareness of vasculature supplying the implanted gestation.77 Surgical treatment is difficult and systemic methotrexate is considered first-line treatment, with an early recourse to more than one dose, for the majority of extratubal ectopic pregnancies.78 A more detailed description of the management of these unusual cases is beyond the scope of this review.
Subsequent pregnancies
Studies suggest that around 60% of women affected by an ectopic pregnancy go on to have a viable IUP.79 This figure includes those who do not plan to have another pregnancy and so the proportion will be higher if further pregnancy is planned. There is thought to be a 5–20% risk of a recurrence of ectopic pregnancy with one previous ectopic pregnancy and a risk of 32% or more following more than one previous ectopic.79 However, the risk is reduced after each subsequent IUP.80 Even when there has been a bilateral salpingectomy there is still a risk of ectopic pregnancy in the interstitial tube or in tubal remnants following IVF. Women should receive an early scan in their next pregnancy to exclude a recurrent ectopic pregnancy.
The future
There have been major advances in the diagnosis and management of ectopic pregnancies during the last 20 years. However, even now a significant proportion of ectopic pregnancies are not diagnosed at presentation and there are wide variations in management strategies between different units. Current screening methods have a high false-positive rate, and are not cost effective. Consequently, there are a number of ongoing studies developing biomarkers that allow definitive diagnosis.53 58 81 In addition, there is a lack of randomised trials investigating the optimal management of ectopic pregnancy, particularly focusing on recurrence rates and impact on future fertility. Results are awaited from a large randomised trial comparing laparoscopic salpingectomy with salpingostomy.82
Acknowledgments
The authors thank Ronnie Grant for graphics support and Dr Graeme Walker for images.
References
Footnotes
-
Funding Andrew Horne receives grant support from the UK Medical Research Council (2009–2013), IKTF (2009–2011) and an Albert McKern Bequest (2010–2011). Colin Duncan holds a Scottish Senior Clinical Fellowship and has grant support from The Cunningham Trust.
-
Competing interests Andrew Horne holds a UK patent for a diagnostic biomarker for ectopic pregnancy (# 0712801.0).
-
Provenance and peer review Commissioned; externally peer reviewed.