Article Text
Abstract
Objectives To describe current co-cyprindiol prescribing in a large, rural general practice in England. To specifically investigate whether co-cyprindiol is prescribed within its license and Medicines and Healthcare products Regulatory Agency (MHRA) guidelines. To investigate the effect of a simple, low-cost intervention on the number and appropriateness of co-cyprindiol prescriptions.
Methods The computerised medical record system in a 17 435 patient general practice was examined to identify individuals prescribed co-cyprindiol. The medical records for each individual identified were examined to see if they satisfied the MHRA guidelines in co-cyprindiol use. Prescribers were then contacted and sent copies of the MHRA guideline. All patients were invited to attend for review. Prescriptions for co-cyprindiol were then re-audited.
Results Co-cyprindiol comprised 3.4% of total combined oral contraceptive prescriptions. The most common indication was acne (69%).
At baseline, the majority of prescriptions did not meet the MHRA guidelines. Prescriptions that did not meet guidelines tended to have been for longer (32 vs 19.5 months). After the intervention, the number of individuals prescribed co-cyprindiol fell (26 vs 12) and the number of prescriptions that met the guidelines increased (30.7% vs 75%). The largest change was a decrease in inappropriate prescriptions for acne.
Conclusions In this population, co-cyprindiol was rarely prescribed, though its use often contravened guidelines. Simple interventions can increase appropriateness of prescribing.
Statistics from Altmetric.com
Introduction
Co-cyprindiol (cyproterone acetate with ethinylestradiol, Dianette®) is a combination oral contraceptive that contains both an anti-androgen (cyproterone) and estrogen (ethinylestradiol). It has been available since 1985 and the manufacturers estimate that there have now been 50 million treatment years of experience with this product. It is licensed in the UK for treatment of women with severe acne refractory to prolonged antibacterial therapy, or as a first line treatment for moderately severe hirsuitism. Medicines and Healthcare products Regulatory Agency (MHRA) guidelines1 state that:
1) Co-cyprindiol should only be used only for its licensed indication
2) It should not be used solely for contraception
3) It should be discontinued 3–4 months after complete resolution of symptoms.
Key message points
▶ Co-cyprindiol was rarely prescribed in this study, but where it was prescribed it was commonly outside of licensed indications.
▶ A simple intervention decreased total co-cyprindiol prescriptions and increased appropriateness of prescribing.
One of the reasons the use of co-cyprindiol has been limited in this way is the concern that co-cyprindiol may be associated with an increased risk of venous thromboembolism (VTE). Though the evidence regarding increased risk of VTE with co-cyprindiol is not clear cut, it is true that other treatments for acne and hirsuitism (and indeed contraception) are available that do not increase the incidence of VTE.2 3
Previous studies have shown that relatively simple and low-cost educational interventions can improve the appropriateness of prescribing in relation to oral contraceptives in UK health care settings.4 Consequently, we were interested in examining the use of co-cyprindiol within our practice, and whether prescribing could be improved via a similar educational intervention.
Aims
The aims of the study were three-fold:
1) To describe current co-cyprindiol prescribing practice in a large (17 435 patients), rural general practice in England
2) To specifically investigate whether co-cyprindiol is used within MHRA guidelines
3) To investigate the effect of a simple, low-cost intervention on the number and appropriateness of co-cyprindiol prescriptions.
Methods
Data collection
On 9 July 2010, the practice Egton Medical Information Systems Ltd (EMIS) computerised database was searched for all patients issued co-cyprindiol within the last 3 months or on repeat prescription. The electronic medical records of each patient were then examined to gather information on length of time prescribed and whether the prescription met the MHRA guidelines. This process was repeated 4 months later, following the intervention, on 9 November 2010. All data collection was conducted by the author. Though this inevitably introduces the possibility of observer bias, it ensured uniformity of approach before and after intervention.
Intervention
All doctors and nurses working within the practice were sent an electronic and paper copy of the MHRA guidelines with respect to co-cyprindiol prescribing with a covering letter explaining the purpose of the audit. All patients identified from the first data collection were sent a letter inviting them to attend the surgery to review their co-cyprindiol prescription with the prescribing doctor. Sixteen (62%) patients made appointments as a direct result of the letter. Three (12%) further patients were seen which resulted in altered prescription, though not as a direct response to the letter.
Results
Co-cyprindiol prescribing outside MHRA guidelines
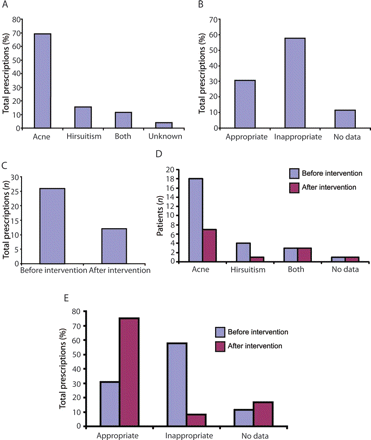
A total of only 26 individuals were prescribed co-cyprindiol (0.15% of all patients registered with the practice, 3.4% of the total of 770 patients prescribed combined oral contraceptives). Of those 26 individuals, the majority, 18 (69%), had been prescribed co-cyprindiol for acne, four (15%) had been prescribed co-cyprindiol for hirsuitism and three (11%) for both hirsuitism and acne. It was not possible to ascertain the reason for initiation in one individual (Figure 1A). Though these are all licensed indications, acne should be both severe and refractory to antibiotic therapy before co-cyprindiol is prescribed. When severity of acne (as opposed to simply whether the individual suffered with acne or not) was taken into account, only eight (31%) prescriptions fulfilled the licensed indication and MHRA guidelines, and 15 (58%) did not, with insufficient data with regards to acne severity or initial indication to reach a conclusion in three (11%) cases (Figure 1B). No prescriptions were initiated for contraception alone. The mean length of time on co-cyprindiol was 28.1 (range 1–120) months. Prescriptions for licensed indications tended to be for shorter lengths of time (19.5, range 1–84, months) than for unlicensed indications (32.3, range 3–120, months).
The indication for, and appropriateness of, prescriptions for co-cyprindiol. (A) Graph shows the percentage of total prescriptions against indication for initiation of prescription. (B) Graph shows percentage of total prescriptions against whether the prescription met the Medicines and Healthcare products Regulatory Agency (MHRA) guidelines (appropriate) or not (inappropriate). Following intervention, the total number of prescriptions for co-cyprindiol fell (C). Following intervention, the biggest decrease seen was in prescriptions for acne (D). The percentage of prescriptions that met the MHRA guidelines (i.e. appropriate) increased following intervention (E).
Decrease in total co-cyprindiol prescriptions following intervention
Following intervention, the total number of people prescribed co-cyprindiol fell (26 vs 12) (Figure 1C). The average time spent on co-cyprindiol was also lower for those individuals still on co-cyprindiol after intervention (mean 28.11 vs 19.25 months).
The decrease in prescriptions was largely accounted for by decreased prescriptions for acne (Figure 1D). The total decrease was entirely accounted for by a decrease in prescriptions outside of license. Unlicensed prescriptions fell from 58% of total prescriptions to 8%, a decrease of 14 prescriptions (Figure 1E).
Discussion
The audit presented here contains a number of interesting and novel findings. It is, to the best of the author's knowledge, the only description of current co-cyprindiol prescribing in UK general practice. That co-cyprindiol is uncommonly prescribed, and in this sample never solely for contraception, was reassuring. There was some evidence it was prescribed for acne that was either not severe enough to merit treatment with co-cyprindiol, or co-cyprindiol was prescribed before other treatments had been tried. We were unable to clearly ascertain whether prescriptions met the third of the MHRA criteria, namely that the prescription should be stopped 3–4 months after complete resolution of symptoms. However, the fact that some individuals had been on co-cyprindiol for 10 years suggests either it had not been discontinued, or that it had been at best only partially effective in controlling symptoms. Inappropriate prescribing decreased following a simple intervention. This adds to the literature suggesting that such interventions can be effective in improving prescribing, and therefore patient care.
Data such as those presented here for a population needs careful interpretation at an individual level. For example, women using a particular method of contraception, perhaps over an extended period, with no problems, may be reluctant to change to a new method. This may lead to decreased concordance and the potential for unwanted pregnancy, if a change in method of contraception is made. For this reason, in this study, individuals were invited to discuss the issue with their own regular doctor. Problems could potentially be further ameliorated by organising follow-up appointments (perhaps by telephone) following any changes to contraception. Long-term outcomes of patients involved in studies such as this, though beyond the scope of the current manuscript, would make an interesting topic for further study.
Acknowledgments
The author wishes to thank Dr Ben Underwood for his help with data interpretation and manuscript preparation, and Alison Couldrey and colleagues at the Christmas Maltings practice for their support and helpful comments.
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.