Article Text
Abstract
Background and methodology Information is presented on the management of women referred to a sexual health service during a 1-year period for management of a non-palpable contraceptive implant or of a palpable implant considered unsuitable for routine removal.
Results Fifty-two women were referred. Thirty-four implants were non-palpable and their depth on ultrasound ranged from 2.7 to 12 mm. Seventeen were fully or partly palpable and their depth ranged from 3 mm or less (16 cases) to 5.6 mm (one case). Nine had had previous failed attempts at removal, including three with two previous failed attempts. Three implants were located at sites other than the medial aspect of the arm, and were associated with long localisation times. Forty-seven implants were removed at a one-stop clinic appointment through a 2–3 mm incision using ring forceps. The mean time for removal from making the incision to complete extraction was 4.8 minutes. Three cases judged to be of higher risk were removed under local anaesthesia in theatre through a 15 mm incision. These included the only case where removal was attempted unsuccessfully in the outpatient clinic. Seven of the implants were Nexplanon®, including four cases seen during the last month of data collection.
Conclusions Removal with the technique described is rapid, with an average time of 4–5 minutes, and less than 10 minutes in >90% of cases. Preliminary indications suggest that the introduction of Nexplanon has not resolved the problem of deep implant insertion. Based on our experience we suggest criteria for centres providing removal of deep implants.
- Implants
- Family Planning Service Provision
Statistics from Altmetric.com
Key message points
Deep implants continue to occur with Nexplanon®. The key to rapid, painless, aseptic removal is ultrasound localisation with accurate marking of the skin, with the arm positioned for the removal.
Using small ring forceps, removal can be achieved through a 2–3 mm incision at any point along the implant without the need to apply pressure to the arm.
The technique is easy to teach, minimally invasive and associated with a high success rate, with an average removal time of under 5 minutes.
Introduction
Despite training and the introduction of Nexplanon®, deep implant placement continues to be a problem. Recent issues of this Journal have contained a number of letters from practitioners giving anecdotal opinions about the merits of their own preferred method of removal.1–,5 Ultrasound scanning is key to the management of these cases. As well as allowing precise localisation, it can be used to demonstrate the proximity to any deep implant of nerves as well as blood vessels. We describe our experience of referrals for deep or failed implant removals during a recent 12-month period.
Methodology
We retrospectively reviewed cases referred to this specialist contraception service during the 12-month period 1 January–31 December 2012. Data were reviewed on age of user, type of device and duration of use, where and by whom it was fitted, whether it was fully palpable, partly palpable or non-palpable, previous attempts at removal, depth and position of implant, method of removal and time taken. Referrals were seen at two sites where ultrasound is available in the clinic. All non-palpable and some palpable implants were scanned and their depth measured at the proximal and distal ends. For cases on one clinic site a curved array 2–6 MHz transducer (generally used for pelvic and early pregnancy work) was used, but with settings adjusted for musculoskeletal imaging. On the other clinic site a small linear array 5–12 MHz transducer was used and generally this gave better distinction of the implant and its shadow despite being a component of a low cost ‘laptop’ scanner. Depth measurement was made with the transducer in light contact with the skin, avoiding any compression of tissues over the implant.
Results
During the 12-month period, 52 women were referred by doctors or nurses providing implant removal in Gloucestershire, UK. In each case the referrer had concluded that the implant was unsuitable for a standard removal technique. In two cases this was because the implant was palpably fractured (Figure 1), in 40 cases it was because the implant was impossible or difficult to palpate, and in nine cases removal had been attempted but had failed. One patient did not attend but had requested another appointment at time of writing. The cases are summarised in Table 1.
Difficult/deep implant referrals during the period 1 January–31 December 2012
Fractured implants and forceps used for removal.
We were able to identify a maximum of three cases within the series for any individual fitter (one doctor and one nurse each fitted three of the implants). Other cases appeared to be single events for the particular fitter and these fitters included some of the most experienced doctors and nurses in our service.
Location of implant
Ultrasound was used to locate all non-palpable implants, and for comparison was also used in some cases where the implant was palpable. In all but three cases the implant was in the medial aspect of the arm. In most cases the lower end of the implant was within 2 cm above a small white scar that appeared to have been the insertion site. However, in one case the implant was close to the axilla with its lower end 5 cm above and medial to the insertion scar, suggesting that the implant had either migrated or that the inserting practitioner had pushed the obturator forward instead of withdrawing the cannula over it. In another case the implant was eventually located in the posterior aspect of the arm close to the olecranon, but only after considerable time had been spent scanning the medial and anterior aspects of the arm trying to locate it. In this case no insertion scar was visible. In a further case the lower end of the implant was 2 cm below the insertion scar and lay just above the medial condyle, suggesting that it had migrated distally.
In nine cases the proximal (upper) end of the implant was 2 mm or more deeper than the distal (insertion) end, suggesting the applicator had been advanced at an angle instead of parallel to the skin surface (Figure 2). The deepest implant was 9.5 mm deep at the insertion end and 12 mm at the upper end. There was no absolute correlation between the depth of implant and palpability. However, only one implant that was more than 3 mm deep throughout was palpable. Surprisingly this palpable implant was 5.6 mm deep in an arm with abundant subcutaneous fat, and was very easy to remove. We did not weigh referred patients or attempt to establish what their weight had been at the time of the implant fitting. It is not our impression that weight gain or obesity makes any significant contribution to deep implant location. We agree with others who believe the problem is poor insertion technique.6 ,7
Transverse images through the lower and upper end of the implant. The upper end is over 7 mm deeper, implying insertion at an angle of around 20° to the horizontal.
Ultrasound was used to ascertain the depth of 43 implants (34 non-palpable, four partly palpable and five completely palpable implants). The depths of the non-palpable implants ranged from 2.7 to 9.5 mm at their most superficial ends. All but one of these were 3 mm or more deep. The non-palpable implant that was only 2.7 mm deep was in a woman who reported that she had lost 3 stone (19 kg) in weight. Removal took significantly longer than average for a non-palpable device, despite it being so superficial, possibly because it had a particularly tough fibrous capsule.
A majority of deep implants lay within 8 mm of a large vessel. In one case the implant appeared to be directly adjacent to the brachial artery and basilic vein in a slim woman (Figure 3). For this reason it was removed in theatre through a longer incision. It was scanned jointly with a radiologist prior to the procedure, using higher specification ultrasound equipment with a high-frequency transducer. Checking the implant's location with the arm in different positions resulted in it appearing closer to or further away from the vessels. This case illustrates that the position of deep implants in relation to skin marking could vary with changes in arm position, either with supination or pronation, or with variation of elbow flexion (Figure 4). Skin marking could therefore become quite inaccurate if the arm is moved between scanning and the start of the removal procedure.
Theatre case 3: implant adjacent to blood vessels.
Removal
One woman elected to leave her deep implant in place after its location had been confirmed on scan. In 48 cases removal was attempted at the time of the outpatient appointment, and this was successful in all but one case. In palpable cases, this was mostly without marking the location demonstrated on ultrasound, but some cases had a depth measurement to allow comparison with non-palpable cases (Table 1). In all non-palpable cases the arm was placed in an appropriate comfortable position for removal and the implant then located using ultrasound. The overlying skin was marked and the ultrasound probe then removed. Taking care to keep the arm position unchanged, the skin was cleaned and 2–5 ml of 1% or 2% lidocaine was injected directly over the marked (or palpable) implant site. Usually the incision was over the shallowest end of the implant, unless a previous attempt had been made in that area or unless it was closer to a large vessel than the deeper end. Usually it was possible to feel the implant with slight sideways movement of the needle once inserted to the appropriate depth. A 2–3 mm incision was made with a disposable No.11 scalpel, and again it was usually possible to confirm the position of the implant with slight sideways movement of the scalpel tip once inserted to the required depth. Owing to skin elasticity a 2–3 mm incision is adequate to allow the 3 mm ring of the forceps (Figure 1) to be pushed through to the required depth. Gentle side-to-side movement of the forceps then usually enabled the implant to be felt, which assisted in opening and closing the forceps with the required precision to enclose the implant. Slight traction would then give confirmation whether the implant was within the ring. Easing the ring back through the incision would allow freeing of any surrounding fibrous capsule with the scalpel or a separate curved mosquito forceps. The small incision was routinely closed with Steri-Strips™. This technique is similar to the ‘U’ technique described for the removal of Norplant® capsules.8
A previous unsuccessful attempt at removal had been made in nine cases, in all of which the implant was fully or partly palpable. Several of these were associated with a scar longer than 5 mm which had been sutured. In three cases two previous removal attempts had been made though the same scar at or beyond a palpable end of the implant. In eight cases the attempted removal incision was at the lower (distal) end and in one case it was at the upper (proximal) end, where this was the only part palpable. No previous attempt had been made with any of the 34 non-palpable implants. In cases with a previous failed attempt the previous incision site was avoided by making the incision for removal over the other end of the implant. Anecdotally several women reported relatively long previous attempted removal times and often complained that the pressure applied to their arm during previous attempted extraction was painful. Many expressed surprise at the ease and speed of removal using the technique described here.
In three cases removal was performed in theatre with local anaesthetic using an open incision technique with a sterile field. These are detailed in Table 2.
Cases of implant removal in theatre
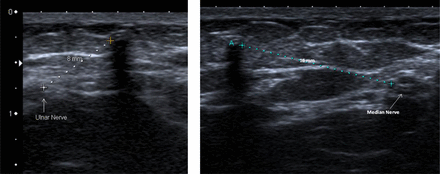
Case 3 was the only case where any abnormal neurological sensation (“like an electric shock”) was reported and for this reason attempted removal in the clinic was abandoned. The implant was scanned with the radiologist prior to the theatre procedure and the median and ulnar nerves localised (Figure 5). The proximity of the ulnar nerve was thought likely to have been responsible for the abnormal sensation during attempted outpatient removal. A more proximal removal site and a longer incision were used in theatre and there was no abnormal neurological sensation.
The theatre cases were undertaken by the first author. In all three cases removal was performed under local anaesthesia, injecting around 5 ml 1% lidocaine, and then making a 15 mm long incision over the implant, just deep and long enough to enable insertion of the tip of the operator's index finger. The skin incision was closed with interrupted 5/0 Vicryl Rapide™. A similar technique was described by Walling with an incision just long enough to insert his little finger.7
Time for removal
Cases referred for deep implant removal were routinely given a 30-minute outpatient appointment. The appointment included discussion with, where appropriate, provision of continuing contraception, localisation of any non-palpable implant and discussion of the option of removal or leaving the implant, as well as the removal procedure. The appointment time allotted was adequate in the majority of cases. We found that locating and marking the position of many of the non-palpable implants generally took longer than the removal process. The longest localisation times were in cases where the implant was in an unusual site as in the three cases detailed above. We timed the removal procedure in 33 cases that were successfully completed in the outpatient clinic. The time of the incision and the time when the removal was completed were noted. The removal time from the incision to complete extraction ranged from less than 60 seconds (two cases) to 10–11 minutes (four cases) with a mean time of 4.8 minutes.
Reasons for removal
In two palpable cases (one Implanon® and one Nexplanon) implant removal was requested because a fracture was palpable in the device. Although advice from the manufacturers is that the contraceptive efficacy is not affected,9 the women did not like the feel of the fractured device and requested removal. In both cases removal with ring forceps was uncomplicated as the devices remained in one piece (Figure 1). In one further case a non-palpable implant was located on scan, but the woman elected to leave it in situ and have a new implant fitted in the other arm. In all other cases the women wished the implant removed either because of side effects, to become pregnant or because the device was time-expired.
Discussion
The most serious complication reported with contraceptive implants is nerve damage.10–14 Such cases have invariably been reported following attempted removal from a deep or non-palpable location, often without ultrasound localisation. There is a lack of awareness that these cases require referral for urgent (within hours of injury) microsurgical repair of the traumatised nerve if permanent sequelae are to be avoided.
In all our cases removal was with local anaesthetic (2–5 ml lidocaine), including the cases of removal in theatre through a longer incision. Such cases took longer but were not associated with any abnormal neurological sensation, which was reassuring, particularly in Case 3 who had experienced abnormal neurological sensation during attempted removal in the clinic. We would always advise patients to have more complex procedures with local anaesthesia because safety is improved if the woman can alert the surgeon to abnormal sensation should the procedure encroach on a nerve. In terms of improving our technique for removal of deep implants, safety could be further enhanced by routinely checking the position of the median and ulnar nerves as well as blood vessels. Vascular structures are easily distinguished as virtually all modern machines have colour Doppler capability. Peripheral nerves may be oval, round or triangular depending on the shape of muscles between which they run. Nerves may run with blood vessels but they generally run along the borders of other structures, especially between muscle groups. They have a variable sonographic echo texture, which depends on surrounding structures. Typically nerve fascicles appear hypoechoic with more hyperechoic perineurium around them, and they run in bundles which when viewed in cross-section may have a honeycomb appearance, like drinking straws end-on. Although ideally visualisation requires a high frequency linear array transducer (9–18 MHz), we found that it is possible with an inexpensive portable machine with a 5–12 MHz transducer.
The technique reported by Mansour et al.,8 using blunt dissection with a curved mosquito forceps and mini-retractors to locate and expose the implant through a longitudinal incision, was tried in the first two cases performed in theatre, and accounted for the relatively long removal times. Once the incision is through the skin, loose fat flopped around the retractors, preventing visualisation. Instead a 1.5 cm linear incision directly over the implant and through the full thickness of the skin enabled insertion of a fingertip and much easier localisation. A non-palpable implant beneath the full thickness of skin becomes fairly readily palpable when a fingertip can be inserted. It was then relatively easy to grasp the implant with the ring forceps and to dissect it free from the fibrous capsule with minimal disturbance of surrounding tissues. Using this technique at the outset resulted in much quicker removal in our third theatre case.
Apart from speed, safety and applicability to all depths of implant, other advantages of this technique are that it is not necessary to apply pressure to the arm, and extraction can be effected at any point along the implant. Patients who had experienced previous failed removal attempts often arrived with an inflamed, incompletely healed incision site. The ability to site a new removal incision well away from the previous incision and the absence of any need to apply pressure to one end of the implant when the arm was sore from previous attempts contributed greatly to the acceptability of the procedure.
We have previously used and would not recommend the technique of ‘needle under the implant’ for several reasons. First, ultrasound location typically shows at least one large blood vessel or nerve nearby, usually just a few millimetres deep to or lateral to the implant (Figures 2⇑⇓–5). These structures would be very vulnerable to puncture by passing a needle under the implant. Second, it is painful and requires a greater area of skin to be anaesthetised. Third, there is high risk of a sharps injury, as commented on recently in this Journal.4 Finally, in the technique described here the implant can usually be felt with the needle during insertion of local anaesthetic and then with the scalpel point, which helps the operator to place the ring forceps precisely, so splinting with a needle underneath is completely unnecessary.
With rotation and flexion of the arm the implant position changes relative to adjacent structures.
Theatre case 3: position of the ulnar and median nerves relative to a deep implant.
A recent letter described use of a transvaginal probe for implant localisation.3 We suggest that this is unwieldy and would make accurate skin marking difficult. It is preferable to adjust the machine setup to focus even a low-frequency transabdominal probe to a depth that will enable visualisation of an implant. In our deepest case the implant was difficult to see with the imaging available in our clinics but was easy to see using a high-frequency linear probe on a higher specification machine in the radiology department. The relationship of the median and ulnar nerves could also be seen easily. This implant was not seen at all on a magnetic resonance imaging (MRI) scan requested by a gynaecologist to whom the woman had been referred. MRI is not a good imaging method for implants and is also not helpful to the removal process. Accurate ultrasound localisation is possible in all cases and allows marking of the most appropriate site for the skin incision, at the safest possible distance away from nerves and blood vessels. We believe that this is the most important step in the management of deep implants. A final procedural point is that we now use lidocaine with adrenaline 1:200 000 for anaesthesia for deep implant removal. We find this preferable to plain lidocaine as it ensures a bloodless field.
Within the UK, regional sites have been established to locate and remove deep and impalpable implants.15 It is unclear what standards qualify an individual or centre to take referrals. On the basis of our own experience we would recommend that the experience, competencies, professional networks and clinical governance outlined in Box 1 should be in place.
Recommendations for standards for units specialising in removal of deep or non-palpable contraceptive implants
A lead professional who deals with at least 12 cases per year.15
Availability of ultrasound with an appropriate transducer (frequency and setup) for visualisation of implants, superficial tissue planes, nerves and blood vessels.
Competence at ultrasound recognition of implants, blood vessels and nerves.
Use of a protocol for clear ultrasound localisation of non-palpable implants followed by marking of the safest site for the skin incision with the arm positioned for the removal.
Access to a musculoskeletal radiologist where there is suspicion that an implant lies within the proximity of a neurovascular bundle.
An agreed pathway for urgent referral to an upper limb surgeon for cases referred with abnormal neurological symptoms following implant insertion, or where there is a suggestion of nerve injury following removal or attempted removal.
A risk management process to identify and feed back information to implant fitters and removers, with recommendation of retraining where multiple systematic errors of practice are identified.
Conclusions
To date it has not been our impression that referrals for localisation and removal of deep implants have started to reduce with Nexplanon, and we wonder if fitters wrongly believe that the newer device will prevent excessively deep insertion. The removal technique we describe is safe, effective and highly acceptable to patients. It has already been our experience that this is an easily teachable skill, provided a very basic level of ultrasound skill and a relatively low-cost imaging device are available. Practitioners should receive training in order to recognise nerves and blood vessels as well as the implant itself. We recommend that recognised centres should have established professional networks and pathways in place to deal with all complications, including access to emergency microsurgical nerve repair in the unlikely event of a nerve injury.
Acknowledgments
The authors wish to thank Dr Matthew Shaw, Consultant Radiologist at Gloucestershire Hospitals NHS Foundation Trust, for assistance with cases unsuitable for outpatient removal and confirming the position of implants and their relation to neurovascular structures.
References
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue