Article Text
Abstract
Aim The aim of this review was to systematically review the outcome of routine anti-D administration among unsensitised rhesus (RhD)-negative individuals who have an abortion. This review is registered with Prospero.
Methods A search for all published and ongoing studies, without restrictions on language or publication status, was performed using the following databases from their inception: EBM Reviews Ovid - Cochrane Central Register of Controlled Trials, MEDLINE Ovid (Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily), Embase.com, Popline and Google Scholar. Study types included: randomised controlled trials, controlled trials, cohort and case–control studies from 1971 onwards. The population included women who undergo an abortion (induced, incomplete, spontaneous or septic abortion), medical or surgical <12 weeks, and isoimmunisation in a subsequent pregnancy. The primary outcomes were: (1) development of a positive Kleihauer–Betke test and (2) development of Rh alloimmunisation in a subsequent pregnancy.
Results A total of 2652 studies were screened with 105 accessed for full-text review. Two studies have been included with high bias appreciated. Both studies found few women to be sensitised in forming antibodies after an abortion. The limited studies available and heterogeneity prevent the conduction of a meta-analysis.
Conclusions Rh immunoglobulin has well-documented safety. However, it is not without risks and costs, is a possible barrier to delivering efficient services, and may have limited availability in some countries. The evidence base and quality of studies are currently limited. There is unclear benefit from the recommendation for Rh testing and immunoglobulin administration in early pregnancy. More research is needed as clinical practice guidelines are varied, based on expert opinions and moving away from testing and administration at time of abortion.
Implications There is limited evidence surrounding medical benefit of Rh testing and immunoglobulin administration in early pregnancy. Further research is needed to define alloimmunisation and immunoglobulin benefit to update standards of care. Additionally, other factors should be considered in forming clinical policies and guidelines such as costs, feasibility and impact on access to care for patients.
- abortion
- incomplete
- abortion
- spontaneous
- abortion
- therapeutic
- abortion
- induced
- reproductive Health
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
Rhesus (Rh) immunoglobulin has well-documented safety but is not without risks, costs or acquisition complexity and may cause unnecessary barriers and delay for patients accessing abortion care.
There is limited evidence on Rh sensitisation for first-trimester abortions at <12 weeks’ gestation (including threatened, spontaneous, surgical or medical abortion).
Clinical practice guidelines currently based on expert opinions vary according to geographical region.
Background
The administration of rhesus (Rh) immunoglobulin was first introduced in 1968 and significantly reduced immunisation to D-antigen.1 Evidence is sparse for such intervention after abortion in early pregnancy.1 Abortion for the purposes of this review included induced (medical or surgical), incomplete, missed, spontaneous or septic abortion at less than 12 weeks’ gestation.
There is a movement to forgo Rh testing and anti-D immunoglobulin administration in people presenting for early abortion, miscarriage or ectopic pregnancy.2 3 For example, the 2019 National Institute for Health and Care Excellence (NICE) guidelines on abortion recommend not offering anti-D prophylaxis to people undergoing medical abortion up to and including 10 weeks’ gestation.4 Rationales to forgo Rh testing and anti-D immunoglobulin administration include lack of evidence that anti-D immunoglobulin prevents Rh alloimmunisation in lower gestational ages and immunoglobulin administration is not without risk as it is a blood product with added costs and care complexity.2 3 For example, added costs and complexity include testing patient blood type and cold storage required for anti-D immunoglobulin.
A systematic review by Karanth et al (2013) examined anti-D administration after spontaneous miscarriage for preventing Rh alloimmunisation with the Cochrane Pregnancy and Childbirth Group.5 Karanth et al concluded there is insufficient evidence available to evaluate the current practices of anti-D administration in unsensitised RhD-negative mothers after spontaneous miscarriage.
To date, there has been one systematic review examining subsequent isoimmunisation after abortion at less than 13+6 weeks’ gestation in 2019.4 In preparation for updating the World Health Organization (WHO) 2012 Safe Abortion guidelines, the objective of the review was to systematically review the effect of routine anti-D administration among unsensitised RhD-negative individuals who have an abortion.
Methods
This review follows Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.6 A protocol for this systematic review is included as online supplemental appendix 1–2. This review is registered with Prospero (CRD42020149073). The population included women who undergo an abortion: induced (medical or surgical), incomplete, missed, spontaneous or septic abortion at less than 12 weeks’ gestation. The intervention examined was routine anti-D administration compared with no anti-D administration.
Supplemental material
A search was performed for all published and ongoing studies, without language restrictions, using the following databases from their inception to 13 May 2021: EBM Reviews Ovid - Cochrane Central Register of Controlled Trials, MEDLINE Ovid (Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily), Embase.com, Popline and Google Scholar (online supplement 1–2). [NB. Popline and Google Scholar were only searched to 23 July 2019.]
Supplemental material
We included randomised controlled trials, controlled trials, cohort studies and case–control studies. The following study types were excluded: case studies, review articles, editorials, letters, advisories, non-comparative studies, unpublished manuscripts, conference abstracts, diagnostic studies, animal studies, cost–benefit analyses, and studies with basic science outcomes. Non-English publications and articles published prior to 1971 were excluded given WHO only started to recommend that Rh testing and treatment with immunoglobulin be made part of the standard protocol of medical care for pregnant women from 1971 onwards.
Primary outcomes included: (a) development of a positive Kleihauer–Betke test (a test that detects fetal cells in the maternal blood) and (b) development of RhD alloimmunisation in a subsequent pregnancy. Secondary outcomes included: (a) detection of atypical blood group antibodies by positive indirect Coombs test after 6 months of exposure (non‐prespecified outcome), (b) need for increased surveillance for suspected fetal blood sampling and fetal transfusions in subsequent pregnancies, (c) neonatal morbidity such as neonatal anaemia, jaundice, bilirubin encephalopathy, erythroblastosis, prematurity, hypoglycaemia (low blood sugar) in subsequent pregnancies and (d) maternal adverse events of anti‐D administration including anaphylactic reaction. Currently, flow cytometry has been found to be a more accurate estimate of sensitisation.1 7 However, this review utilised the Kleihauer–Betke test as an inclusion criterion because it is more available and commonly used in clinical settings.
Three authors (MCC, CRK and RKG) independently screened all the titles, abstracts and full texts identified from the initial search to determine eligibility for inclusion. The intervention examined was routine anti-D administration compared with no anti-D administration. Studies that did not explicitly include routine anti-D administration as the intervention were excluded. Wrong comparators would include the use of different testing for the primary outcome of developing RhD alloimmunisation confirmed with a positive Kleihauer–Betke test. Conflicts were resolved through discussion and consensus first between MCC and RKG and, if further discussion was needed, with the third reviewer CRK. We used a standard template for data abstraction (table 1).
Rhesus (Rh) isoimmunisation in unsensitised Rh-negative individuals seeking abortion at <12 weeks
Data synthesis
A narrative synthesis was performed; the findings are summarised based on study design, population, intervention, comparison, results, strengths and weaknesses based on information reported by the study authors and subjective assessment of the review authors (table 1). The limited number and heterogeneity of the included studies prevent the conduction of a meta-analysis. Risk of bias was assessed by MCC and CRK with quality of evidence assessment frameworks. Cochrane was used for randomised trials and ROBBINS-I tool for observational studies.8 9
Results
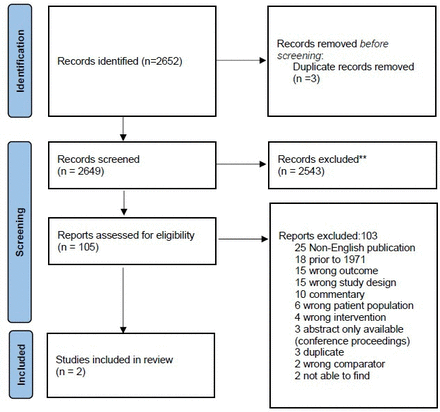
The search strategy identified 2652 publications and three duplicate records were removed, leaving 2649 publications for abstract screening (figure 1). From abstract screening, 105 publications underwent full-text review. The two included studies were cohort studies taking place in the United States and Israel.10 11 Both studies included women with induced abortion in addition to other types of abortion.
PRISMA flow diagram.
A 1972 study funded by Ortho Research Foundation and the Kaiser Foundation Hospital performed a prospective double-blind study collecting data on 491 women undergoing therapeutic abortions between 1 November 1969 and 15 August 1970 in California.11 The primary aim of this study was to investigate Rh immunisation incidence in a postabortion period of 6 months or more in RhD-negative people. From the 491 therapeutic abortion patients, and 180 spontaneous abortion patients, 57 were RhD-negative based on indirect Coombs at the time of abortion management. The resulting sample size was 44 because three refused participation, nine were lost to follow-up and one husband was found to also be RhD-negative (paternal genotyping was only performed in 50% of couples). Within 72 hours of abortion the patient received RhoGAM or a placebo. The study found two of 36 sensitisations in the placebo group with indirect Coombs positive after abortion. All patients receiving RhoGAM were not found to be sensitised. Despite a ‘double-blind’ approach there was no description of allocation concealment, therefore the authors of this review considered this an impact on study quality. However, the objective measurement of outcome (titres) is a strength of the study. Other factors contributing to the limitations of the study and its quality include: no description of allocation, no follow-up rate reported for patients, no defined sample size, no power calculations for sample size, no intention to treat analysis and no report of a ‘Table 1’ for the demographics of the population studied.
The second included study was published in 1972 and followed 170 RhD-negative postabortion patients for 6 months or more with antibody titre testing.10 The primary aim of this study was to identify Rh sensitisation after an abortion in those who did and did not receive RhoGAM. There was no clear description of data collection duration. For example, patients were followed for different times depending on their antibody status. All postabortion participants were observed for 6 months with antibody titres performed at 6 weeks, 3 months and 6 months. In sensitised participants with antibodies detected at 3 months, repeat follow-up serology was performed 24 months after abortion. The study followed patients identified as RhD-negative mothers without antibodies in their blood and a negative Coombs test. When possible, a husband’s ABO blood group was also determined. There were 48 in the intervention group, receiving an injection of RhoGAM, and 122 in the control group. The main outcome was incidence of rhesus sensitisation in a period of 6 months or more postabortion in RhD-negative mothers. The study identified five mothers who became sensitised in the control group. There were no sensitisations found in the intervention group. The authors of this review assessed the limitations of the study and its quality which included: patients not randomised (patients were placed in the control group if RhoGAM was not available), lack of allocation concealment, lack of reported follow-up rate, no sample size or power calculation. The study did not identify sensitised patients within the intervention group.
Discussion
Our review identifies limited evidence on the effectiveness of anti-D administration in women who are RhD-negative and undergo an early abortion. This is consistent with past literature reviews which also provided limited results.1 12 13 Our findings are consistent that there are few studies showing maternal sensitisation or fetal haemolytic disease due to feto-maternal haemorrhage from first-trimester abortion.1 12 13 Furthermore, evidence surrounding sensitisation secondary to ectopic pregnancy is very limited.13
There is no evidence of clear benefit in early pregnancy or the gestational age at which sensitisation can occur among aspiration or medication abortion.2 3 Current clinical practice guidelines are generally based on expert opinions.4 14 The prevalence of RhD-negative varies among populations.15 A review comparing Canadian and Netherlands policies surrounding Rh immunoglobulin administration in first-trimester pregnancy found no differences in sensitisation.2 The populations between Canada and the Netherlands were found to be similar, however there is a difference in practice: Canada recommending Rh immunoglobulin administration and the Netherlands, at the time of this study, offering Rh immunoglobulin to RhD-negative women having spontaneous abortions over 10 weeks 0 days’ gestation and induced abortions over 7 weeks 0 days.2 Furthermore, in the Netherlands, where no anti-D immunoglobin is provided routinely in early abortion, showed lower sensitisation rates than Canada.2 There does not appear to be a clear benefit in Rh immunoglobulin administration in the first trimester.
A recent prospective study examined 42 pregnant people at 5–12 weeks’ gestational age for the minimum fetal red blood cell concentration required to cause maternal Rh sensitisation.16 The study found fetal blood cell exposure in the first trimester to be well below a calculated threshold for Rh sensitisation.16 Of note, the study used flow cytometry which is a more accurate marker of feto-maternal haemorrhage.16 However, the authors identified that larger studies are needed to confirm their study findings.16 These findings also support the suggestion there is no advantage in Rh immunoglobulin administration in the first trimester.
This review is robust from the systematic search of the literature. However, the studies included for this review were of poor quality.10 11 Included studies are quite dated whereby flow cytometry is now considered the most accurate method of testing.1 Given this, it is important to consider changes in clinical practice and standards contextually. For example, previous surgical techniques likely included more sharp curettage relative to today’s standards. Additionally, the included studies had small sample sizes, with approximately 50 women in each study.
Given minimal quality evidence and no clear benefit of RhD immunoglobulin administration after early abortion, and some disadvantages of doing so, further research is needed. Some disadvantages to the routine administration of RhD immunoglobuin include: cost, cold storage, and barriers to access in the context of increasing self-managed abortions. There is also a dissonance surrounding clinically used versus laboratory research measurements of isoimmunisation. For example, the Kleihauer–Betke test is widely available and clinically used in contrast to new evidence that supports flow cytometry as the most accurate method of assessment. A more robust study should utilise flow cytometry to define sensitisation. Included studies in this review utilised the Kleihauer–Betke test which is more accessible and practical in clinical settings compared with flow cytometry. Up-to-date studies with accessible routine laboratory methods and standard definitions for sensitisation or follow-up times to appreciate sensitisation are needed rather than referring to dated studies with limited quality, such as the ones included in this review. In the process of reinforcing standards of practice, practical guideline application is needed for potential global clinical practice change. This would include considerations not limited to: (a) anti-D administration logistics and practicalities, (b) access to instruments or tools to appreciate sensitisation and (c) access, including people self-managing abortion, as medical abortion is becoming more prevalent and thus do anti-D immunoglobulin recommendations help or hinder access to safe abortion care.
Conclusions
Rh immunoglobulin has well-documented safety. However, it is not without associated risks and costs, and it introduces barriers to accessing care that may impact especially on populations with restricted access (ie, legal, financial or resource-wise). The evidence base and quality of studies are currently limited, but there is some reassurance and experience from several national guidelines to justify no longer requiring its use in the first trimester. There is unclear benefit from the recommendation for Rh testing and immunoglobulin administration in early pregnancy. Clinical practice guidelines are based on expert opinions which are varied and moving away from testing and administration at the time of abortion. Therefore, more robust research would help give the reassurance needed to achieve substantial changes to guide clinical practice.
Ethics statements
Patient consent for publication
Acknowledgments
The authors appreciate the help provided by Robyn Paynter, Cochrane Fertility Regulation Group, in the development of the search strategy. They also appreciate librarian assistance from the College of Physicians and Surgeons of British Columbia.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @chanmichellec
Contributors Three authors (MCC, CRK and RKG) independently screened all the titles, abstracts and full texts identified from the initial search to determine eligibility for inclusion. Conflicts were resolved through discussion and consensus first between MCC and RKG and, if further discussion was needed, with the third reviewer CRK. Risk of bias was assessed by MCC and CRK.
Funding UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP)
Disclaimer The authors alone are responsible for the views expressed in this review article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.