Article Text
Abstract
Objectives Health care costs are one of the greatest challenges in modern medicine. In gynaecology, diagnosing and excluding ectopic pregnancy (EP) has been shown to be a financial burden to health services because it commonly requires multiple investigations and hospital visits. However, the full economic costs are not captured by an analysis of health care costs alone. This study therefore aimed to assess the indirect costs to patients of diagnosing and excluding EP.
Methods Patients presenting to a Pregnancy Support Centre in a large UK teaching hospital with abdominal pain and/or bleeding and a positive pregnancy test were recruited during the period June 2010–February 2011. Patients were provided with questionnaires to be completed at home and designed to record and quantify costs that they had incurred until a final diagnosis of their condition was made. A cost–description analysis was performed.
Results 52/203 (26%) recruited patients returned completed questionnaires. The mean cost to patients of diagnosing or excluding EP was £135.13±£51.60 (median £20.70). The main cost drivers identified were hospital visits, holiday cancellations, income loss and household help.
Conclusions Quantification of the indirect costs of diagnosing and excluding EP is challenging because it relies on questionnaire feedback from patients at a time when they have suffered from the emotional impact of pregnancy loss. However, initial estimates suggest that such costs are significant due to diagnostic delays. This further highlights the importance of the development of potential biomarkers of EP to allow prompt diagnosis.
- health economics
Statistics from Altmetric.com
Key message points
-
Diagnosing and excluding ectopic pregnancy (EP) using currently available diagnostic tools requires multiple hospital visits, particularly if the final diagnosis is confirmed to be EP.
-
Diagnosing and excluding EP using currently available diagnostic tools represents a significant personal financial burden for patients.
-
The main cost drivers are expenses due to hospital visits, holiday cancellations, household help and income loss.
Introduction
The costs of health care are considered to be among the greatest challenges in modern medicine. However, full economic costs are not captured by an analysis of health care costs alone. The costs of care (e.g. medication, clinic visits or hospitalisation) are direct costs. Indirect costs are incurred through reduced productivity, reduced educational attainment, and costs associated with other consequences such as travel. While indirect costs are challenging to quantify, they are critical for informing public policy decisions.
In gynaecology, the diagnostic process of diagnosing and excluding ectopic pregnancy (EP) results in significant direct costs1–3 but little is known about indirect costs. Fewer than 50% of EPs are diagnosed at first presentation and patients experiencing pain and/or bleeding in early pregnancy commonly require multiple investigations and hospital visits until a diagnosis for their symptoms can be established.2 ,3 We hypothesised that this may have a significant socioeconomic impact on the patients and their social and working relations. The aim of this study was therefore to perform a descriptive analysis of the cost of diagnosing and excluding EP from a patient perspective.
Methods
Study design
This study used a questionnaire designed specifically to collect information on the personal costs incurred by patients undergoing investigations to obtain a diagnosis for pain and/or bleeding in early pregnancy. First, a focus group discussion (FGD) was held involving nurses, doctors and patients at the Pregnancy Support Centre (PSC) at the Royal Infirmary in Edinburgh, Edinburgh, UK. The FGD aimed to identify possible health care and non-health care cost items as well as productivity loss items. Questions to evaluate the identified costs groups were then developed based on outcomes of the FGD and on existing literature.4 ,5 Their design was aided by using established tools such as the Work Productivity and Activity Impairment Questionnaire.6 The questionnaires were piloted with five patients, reviewed for fact and content validity by the authors and the patients themselves, and final versions were subsequently prepared.
Patients were invited to record time spent and expenses incurred during visits to their general practitioner (GP), the PSC and during hospital admissions. Specific probes included questions about direct income loss, medication and sanitary product purchases, help at home, holiday cancellations and time lost from education. In addition, the questionnaires aimed to measure the possible effect of the current diagnostic pathway on their relationships. Demographic data and income group data5 were also collected. Cost descriptions were made in 2010–2011 estimates using £ sterling as currency.
Recruitment and data collection
Women presenting to the PSC with a positive pregnancy test and abdominal pain and/or vaginal bleeding between 1 July 2010 and 28 February 2011 were invited to take part. Sampling was purposive and non-randomised.
Patients who had been given a diagnosis on the day of recruitment were provided with one questionnaire. These patients were asked to recall any personal costs incurred during the diagnostic process.
Patients who had not received a diagnosis on the day of recruitment received two questionnaires: one in which they were asked to recall any personal costs incurred up until and including the day of recruitment, and the other in which they were asked to prospectively record data on personal costs incurred until they were given a definite diagnosis for their symptoms.
All patients were provided with pre-labelled and franked envelopes and were asked to post back all completed questionnaires. To ensure anonymity each questionnaire was labelled with a unique identifier number.
In view of the likely significant emotional impact of pain and bleeding in pregnancy the investigators opted to refrain from contacting patients to supplement data gaps or to track missing questionnaires.
Data analysis
Statistical analysis was performed using STATA 10 (StataCorp LP, College Station, TX, USA) and EpiInfo7 (CDC, Atlanta, GA, USA).
Results
A total of 203 patients was recruited, of whom 52 returned their questionnaires (a 26% response rate). The causes of symptoms in respondents included EP (n=9), miscarriage (n =25), ongoing pregnancy (n =16) and others (ovarian cyst, n =1; twin pregnancy with demise of one twin, n =1). In comparison, causes amongst non-responders (data available for 148/151 patients) were EP (n =11), miscarriage (n =62), ongoing pregnancy (n =71) and pregnancy of unknown location (n =4), with more EPs and fewer ongoing pregnancies in the responder group [χ2=6.74, p<0.05, df=2; Chi-square (χ2) test].
Total costs to patients
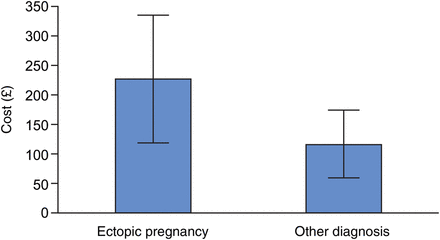
Amongst respondents, the mean patient cost of diagnosing and excluding EP was £135.13±£51.60 (median £20.70) (Table 1). Patients with a final diagnosis of EP incurred on average more costs compared to those with a diagnosis other than EP (Figure 1), although this difference was not statistically significant (n=52; t-test for unpaired data: t(50)=0.8143, p>0.05). Direct health care costs (Table 1) included purchases of medication for pain relief. None of the respondents stated that they required informal care from family or friends. Direct non-health care costs (Table 1) included transportation costs to the GP, the PSC and to the hospital ward (fuel, bus and taxi fares, parking); costs of support with household activities; and purchase of sanitary products. The number of PSC visits ranged from one to seven (total of 132 visits required) and each patient required on average more than two visits to the PSC. This number was similar amongst non-respondents (data not shown). When combining data for respondents and non-respondents, and focusing on EP, miscarriage or ongoing pregnancy, there was a significant difference in mean number of visits depending on the final diagnosis [n=194; one-way analysis of variance (ANOVA): F(2191)=7.08, p<0.01]. A Bonferroni subanalysis showed that there was a significantly higher mean number of visits for EP and miscarriage than for ongoing pregnancy, but no significant difference between EP and miscarriage. Of note, 14/52 (27%) patients stated that they had had to cancel a planned holiday, of whom eight lost money because of the cancellation (an average of £388.13±£186.96 was lost for each of these patients, median £147.50) (Table 1).
Summary of costs in £ sterling incurred by patients presenting with lower abdominal pain and/or bleeding and a positive pregnancy test
Mean total cost (± standard error) in £ sterling incurred by patients with a final diagnosis of ectopic pregnancy (EP) (n=9), compared to mean total cost of those women who had EP excluded (n=43).
Indirect costs of productivity loss and opportunity cost
Information on occupation and income was available for 51 respondents, of whom 44 (86%) were earning a monthly salary (39 were employed, five self-employed), working on average 32 (±1.5) hours per week (n=41). A total of 34/52 (65%) patients stated they had missed time at work because of their symptoms prior to a diagnosis and the calculated mean time lost at work for all respondents was 31 hours. Of those, seven (14%) patients had a direct income loss (mean £377.34±£277.85, median £95.00) (Table 1). Patients admitted to hospital in order to obtain a diagnosis for their symptoms did not record an income loss. In order to assess productivity loss, participants were asked to complete 10-point visual analogue scales (0=no loss, 10=total productivity loss) that assessed productivity loss due to their symptoms while working (n=36) and while doing other regular activities outside work (n=49). For respondents, the mean score for productivity loss at work was 4.56 (median 5, mode 0, range 0–10), and the mean score for productivity loss relating to non-work regular daily activity was 4.12 (median 4, mode 5, range 0–9). Only one respondent stated she had lost time in education due to her symptoms (3 days).
Intangible costs
Of 52 respondents, 13 (25%) confirmed that the diagnostic process had affected their relationship, leading to increased stress levels and anxiety, which at times had led to arguments and to cessation of sexual intercourse.
Discussion
This study demonstrates that the personal financial cost of diagnosing and excluding EP to Scottish women is significant and close to £135 per diagnostic episode. The main cost drivers detected are due to diagnostic delay and include hospital visits, costs for household help, costs due to holiday cancellations and actual loss of income. In addition, we found that the diagnostic delay can lead to relationship strain.
These findings are important because they further underline the importance of developing potential biomarkers of EP to allow prompt diagnosis.6 In a previous study, we estimated that use of a theoretical point-of-care diagnostic biomarker could reduce the cost to the health care system of diagnosing and excluding EP by up to 70%.3 These new data suggest that the savings to society as a whole could be even higher when accounting for personal financial costs and the possible emotional impact of a prolonged diagnostic process.
Nonetheless, these findings have to be interpreted in the light of the study's limitations. First, the questionnaire return rate was comparatively low (26%), highlighting the challenge of having to rely on questionnaire feedback from patients at a time when they have suffered from the emotional impact of pregnancy loss. Second, respondents and non-respondents had a differing outcome distribution (with higher proportions of EP and miscarriage in the responder group) and respondents did not include many ethnic minority patients. Both the difference in outcome distribution and lack of ethnic minority patients in the sample may reduce the validity of findings, and extrapolation of these figures to a larger Scottish population would need to account for this. Third, patients were partly required to document expenditures retrospectively, introducing recall bias with the potential to both overestimate and underestimate cost. Lastly, it is likely that there was some under-reporting of costs, and indeed for most cost items there was a discrepancy between those individuals who noted that they had required a hospital visit and those who documented costs incurred for that visit.
In conclusion, we believe that this study demonstrates that the health care provider/National Health Service perspective generally adopted when determining cost-effectiveness may not be sufficient and that personal costs to patients must be taken into account. Thus, further research in EP diagnosis should focus on evaluating financial costs and impact on quality of life in more detail and in a larger population. These data would then have the potential to be used in a cost-comparison analysis comparing current diagnostic methods with a theoretical single-visit diagnostic biomarker of EP with high sensitivity and specificity.
Conclusion
The present study has demonstrated that while the diagnosis and exclusion of EP is expensive and time-consuming for the health care provider, patients themselves carry a significant personal financial burden.
Acknowledgments
The authors would like to thank Dr Olivia Wu (Health Economist, University of Glasgow), the staff of the Pregnancy Support Centre at the Royal Infirmary of Edinburgh, Ann Doust and Catherine Murray for their invaluable help with the study.
Footnotes
-
Funding Dr Duncan is supported by a Scottish Senior Clinical Fellowship and Dr Horne is supported by a MRC Clinician Scientist Fellowship (G0802808).
-
Competing interests Drs Horne and Critchley hold the patent ‘Identification of Ectopic Pregnancies’ #0712801.0.
-
Ethics approval Ethical approval was obtained from the South East Scotland Research Ethics Committee in April 2010 (LREC approval 10/S1103/5).
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue