Article Text
Statistics from Altmetric.com
I would l like to bring to the attention of journal readers a serious safety issue with the TT380 Slimline device due to failure of the device to release into the uterine cavity.
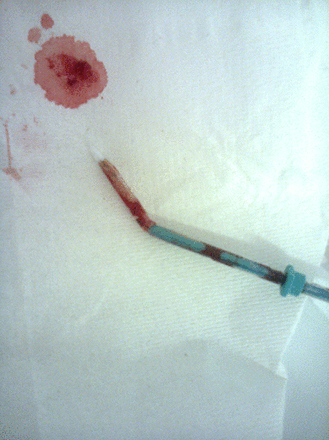
After full counselling the patient chose a copper intrauterine device (IUD) for long-term contraception. Pelvic examination was normal and the uterus sounded to 8 cm with use of a sound and tenaculum. The uterus was mobile and retroverted with a marked angulation at the uterocervical junction, which left the plastic sound gently curved at about 4 cm from the external cervical os. The device was loaded correctly into the inserter and passed into the cavity to the fundus. However, when release of the device was attempted it was impossible, even with attempts to straighten the canal using traction on the tenaculum. On abandoning the procedure the device appeared as shown in Figure 1. The insertion tube had kinked just between the end of the IUD and the tip of the internal plunger, which had perforated the insertion tube (Figure 2).
Introducer rod has perforated the outer inserter tube and unable to release the device due to the sharp bend in the introducer tube.
Intrauterine device still within the distal part of the inserter tube, with the inserter stuck in the proximal part and prevented from moving by the kinked tube.
Testing the insertion tube after removal it was found to kink very easily when slightly bent.
I suggest that the material used for the insertion tube of this device is insufficiently flexible for safe insertion into a uterus with sharp angulation between the uterus and cervical canal. I consider it important for future patient safety that this incident is available for colleagues fitting IUDs to consider and respond to, if appropriate.
Footnotes
Competing interests None declared.
Provenance and peer review Not commissioned; internally peer reviewed.
Linked Articles
- Letter to the editor