Article Text
Statistics from Altmetric.com
Patient
Michelle, a 49-year-old nurse, attends your clinic for a discussion about the menopause. She describes having hot flushes and night sweats, frequent migraines, disturbed sleep, anxiety and poor memory. She is using a 52 mg levonorgestrel-releasing intrauterine system (LNG IUS 52 mg) to control her heavy menstrual bleeding and has not had a ‘period’ for several years. Her mother was diagnosed with breast cancer when she was 55.
Intervention
Michelle is keen to explore HRT.
Comparison
She has tried sage (over the counter) with little benefit and is interested in knowing more about alternative therapies such as bioidentical hormone replacement therapy (HRT) as well as standard HRT.
Outcome
Improvement in vasomotor symptoms and migraines.
Key messages
Migraine can be triggered in the perimenopause by fluctuating oestrogen levels and vasomotor symptoms. Migraine with aura is not a contraindication to transdermal HRT and may reduce headache frequency.
Many women enquire about alternative therapies including compounded ‘bio-identical’ HRT. These products are unlicensed, unregulated with limited evidence supporting efficacy and safety. ‘Body-identical’ HRT however, (transdermal oestradiol and oral micronised progesterone) can be prescribed and effectively treats menopausal symptoms.
NICE guideline on familial breast cancer outlines situations where women with a positive family history but without a personal history of breast cancer, can be cared for in primary care and receive HRT.
Patient
This case vignette illustrates a common presentation of perimenopausal women demonstrating the importance of individualising care to meet each woman’s needs. All women experience menopause, yet symptoms vary and making a diagnosis is challenging, particularly if they are using progestogen-only contraception. The average age of the menopause in Europe is 51 years, but this may be influenced by genetic, medical and environmental factors.1
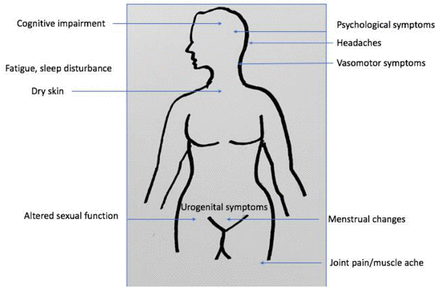
The perimenopause is a time of transition, with menopausal symptoms (see figure 1) often starting 1–2 years prior to the last menstrual period. Symptoms may last on average 7 years with 20% of women still experiencing flushes and sweats for 15 years.2 Michelle reports worsening migraines but also problematic vasomotor symptoms as well as a poor sleep pattern, episodes of anxiety and poor concentration. We asked Michelle to rank her symptoms in order of severity. Validated menopause symptom questionnaires are available to rate and quantify severity of symptoms, but these may not be practical in clinical practice where consultation time is limited. Michelle’s main concerns are flushes and worsening migraine headaches, probably related to fluctuating hormonal levels during the perimenopause.
Menopausal symptoms
Michelle has two children and no other relevant past or present medical problems. On further exploration of Michelle’s migraine history, she describes migraine with aura, for which she manages her symptoms with triptans and simple analgesia.
Michelle is concerned about breast cancer as her mother developed breast cancer at the age of 55; no other family member has been affected. The NICE guideline on familial breast cancer3 suggests that ‘people without a personal history of breast cancer can be cared for in primary care if the family history shows only one first-degree or second-degree relative diagnosed with breast cancer at older than age 40 years (in most cases, this will equate to less than a 3% 10-year risk of breast cancer at age 40 years), provided that none of the following are present in the family history: bilateral breast cancer, male breast cancer, ovarian cancer, Jewish ancestry, sarcoma in a relative younger than age 45 years, glioma or childhood adrenal cortical carcinomas, complicated patterns of multiple cancers at a young age or paternal history of breast cancer (two or more relatives on the father’s side of the family)’.
A psychosocial history may help to identify relevant lifestyle factors or environmental triggers to be addressed or explored further. These may include external stressors that affect sleep pattern, anxiety levels and alcohol consumption plus advice related to weight-bearing exercise, nutrition and normalising weight. Michelle is drinking 21 units of alcohol per week, and is aware that this is more than the recommended weekly intake. She has also gained 5 kg over the past year. She is feeling anxious, with interrupted sleep, resulting in her feeling fatigued and forgetful at work.
A medication history should include ‘over the counter’ as well as prescribed medicines. Some complementary therapies may be harmful, such as black cohosh which is linked with liver injury, Chinese angelica which has coumarin activity or St John’s wort which is a liver enzyme-inducing drug. Michelle is not on any regular medication but is taking sage.
Michelle asks to have a blood test to confirm that she is menopausal. We explain that routine laboratory testing to diagnose the menopause is not recommended in women over 45. Follicle stimulating hormone levels can fluctuate during the perimenopause and are therefore unreliable.4 Furthermore, gonadotrophin levels should not guide decisions on stopping contraception because return of menstruation and ovulation may occur resulting in pregnancy (see table 1).4 Where clinically indicated, secondary investigations may be appropriate, such as thyroid function, screening for diabetes, lipid profile and bone densitometry. This is also an opportunity to highlight the importance of engagement in national cancer screening programmes for the breast, cervix and bowel.
When contraception can be discontinued
Postmenopausal, intermenstrual and postcoital bleeding should always be investigated according to local guidelines. This is particularly important in those at higher risk of endometrial pathology (women who are obese, have diabetes, take tamoxifen, have polycystic ovary syndrome or are nulliparous)5 (see table 2).
Management of postmenopausal, intermenstrual and postcoital bleeding (adapted from local guidelines)
Prior to discussion of treatment, body mass index (BMI) and blood pressure should be recorded. Michelle has a BMI of 32 kg/m2 and is normotensive. Hypertension should be controlled if women wish to start or remain on HRT in order to reduce known associated risks such as myocardial infarction or stroke.1
Intervention
Michelle is keen to explore HRT but, in view of her family history, thinks it is contraindicated. The British Menopause Society guidance says there is no strong evidence to suggest that HRT has an additive effect in women with a higher personal risk of breast cancer.6 NICE also says that if a woman does not fulfil the criteria to be referred to secondary care in relation to her family history, HRT may be prescribed.3 Although there are many situations where women may choose not to take HRT or clinicians would advise the use of non-hormonal alternatives first, there are few absolute contraindications to HRT. The decision to use HRT is a woman’s individual choice following a fully informed discussion. Michelle is reassured by this information.7
Consultation time is limited so you may wish to direct women to the following websites: www.menopausematters.co.uk, www.womens-health-concern.org or wwwthebmsorguk. Information-giving will help women to understand the stages of menopause, common symptoms, and associated long-term health implications. Exploring treatment options in a holistic manner and individualising care is the key to successful management. Counselling women about the comparative lifestyle risk factors for breast cancer versus HRT (excess breast cancer risk in women who drink ≥2 units of alcohol per day, smokers, women with a BMI ≥30 kg/m2 and reduction in breast cancer risk in women who undertake at least 2.5 hours of moderate exercise a week) can help decision making and support women to address lifestyle factors where relevant. Addressing lifestyle factors (sleep hygiene, exercise, diet/weight loss, smoking cessation, avoidance of alcohol, caffeine, spicy food and warm environments) is first-line management as supported by NICE.8 Michelle is motivated to engage in regular exercise and will download MyFitnessPal app to assist with weight loss too.
HRT is the most effective treatment for vasomotor symptoms, reducing flushes and sweats by up to 87%.9 The type of HRT is determined by menopause stage, presence or absence of a uterus, risk factors, medication and patient preference. First-line HRT should be prescribed at the lowest dose required for symptom control. Where there are risk factors for venous thromboembolism and stroke, transdermal HRT is preferred.8 10 Oestradiol patches, gels and spray are also advised in those suffering from oestrogenic side effects with oral HRT (fluid retention, bloating, nausea, headaches, breast tenderness, leg cramps), those taking enzyme-inducing drugs (eg, St John’s wort), and those with BMI >30 kg/m2, malabsorption, liver disease or migraine.1 Michelle was told that she could not take a combined contraceptive pill because she suffered from migraine with aura. We assure her that transdermal HRT can be tried. Many clinicians increase the dose of oestrogen slowly over the first few weeks to reduce the chance of migraine headaches developing.
Michelle is reassured to hear that perimenopause can exacerbate migraines11 and transdermal oestradiol plus her LNG-IUS 52 mg may help. Currently, Mirena is the only LNG-IUS 52 mg with a licence to provide protection against endometrial hyperplasia during oestrogen therapy. It will need to be changed every 5 years if oestrogen replacement therapy is prescribed4 and allows perimenopausal women to have a period-free HRT formulation. Otherwise, those who have had a period within the previous 12 months require sequential HRT (continuous oestrogen and 10–14 days of progestogen). Continuous combined HRT (CCHRT) is prescribed to those more than 12 months from their period, no matter what their age. CCHRT avoids a monthly bleed and may reduce the risk of endometrial cancer.12
HRT may have additional benefits on cognition, mood, fatigue and libido as well as local effects on the urogenital tract. Long-term benefits include protection against osteoporosis, improving muscle strength and joint ache.1 There is some evidence that HRT reduces the risk of bowel cancer.13 Recent studies suggest that HRT may prevent cardiovascular disease if started within 10 years of the menopause. This ‘window of opportunity’ may reduce the incidence of cardiovascular disease by approximately 50%.14 If HRT is started (or restarted) in women over 60, the risk increases. This is presumed to be due to the accumulation of atherosclerosis in intervening years which may be destabilised by HRT. Also, all other risks of HRT increase with increasing age.
Comparison
Michelle is interested in knowing more about alternative therapies. The ‘whole body’ holistic approach to managing the menopause and improving well-being should not be underestimated, although robust clinical data are lacking. Approaches may include yoga, meditation, homeopathy and reflexology. Where women have anxiety or low mood related to the menopause, cognitive behavioural therapy should be considered.
Michelle enquires about ‘bio-identical’ HRT and we advise that compounded bio-identical HRT is unlicensed and unregulated with limited evidence supporting efficacy and safety.8 ‘Body identical’ HRT (transdermal oestradiol and oral micronised progesterone), which is prescribed and regulated, is discussed and its benefits explored.
Topical oestrogen is a good option for treating symptoms of vulvovaginal atrophy even if women are taking systemic HRT.15 A recommended maintenance dose can be used long term with users asked to seek help if they experience any vaginal bleeding. Systemic progestogens are not required, nor monitoring of the endometrium because of the low systemic absorption and there is no evidence of increased breast cancer risk associated with the use of topical oestrogen.1 Vaginal moisturisers and lubricants can be used alone or in addition. For women requesting treatment for low libido, aside from HRT, tibolone (a gonadomimetic drug which acts as a systemic oral HRT with androgenic effects) or testosterone supplementation (unlicensed) may be considered.16 In many areas of the UK it requires a specialist to initiate testosterone and this can then be continued in primary care once a maintenance dose has been achieved. There are no long-term safety data and the potential for side effects should be discussed.
Michelle takes sage but is unsure if it is offering any symptom relief. Although popular, there is no good evidence supporting the use of sage.17 We also say that the efficacy and safety of complementary therapies are unknown, may be associated with serious adverse effects and are not supported by NICE.8 17 Phytoestrogens (eg, soy, red clover) are plant-based substances with effects similar to oestrogen acting like hormone regulators. These should be avoided in women with hormone-dependent cancers or those taking tamoxifen. Evidence is conflicting on their effect on vasomotor symptoms.18
Limited data suggest that progestogens (10 mg medroxyprogesterone acetate once daily or megestrol acetate 160 mg once daily) may be effective in controlling vasomotor symptoms where HRT is contraindicated.19 Other medical treatments (selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), clonidine, gabapentin, oxybutinin), of which clonidine is the only licensed non-hormonal treatment for vasomotor symptoms, may be tried in women unable to take HRT and have bothersome flushes and sweats. Evidence concerning efficacy is conflicting and they should not be prescribed first-line where there are no contraindications to HRT unless this is the woman’s preference.1 HRT can be considered to manage low mood associated with the menopause, with the use of SSRI/SNRIs reserved for women with a clinical diagnosis of depression.8 Recognising chronic depression is important to enable onward referral for support where required or adequate surveillance is put in place.
It is thought that the hypothalamic neuropeptide, neurokinin B, along with its receptor is involved in the central signalling of vasomotor symptoms. Initial human studies have suggested that oral neurokinin-3-receptor (NK3R) antagonists are effective in controlling menopausal flushes and sweats, and are well tolerated. Several NK3R antagonists are now being further investigated in phase 3 studies.20 Future developments requiring further research include stellate ganglion blockade to reduce vasomotor symptoms in patients with breast cancer,21 and the use of dehydroepiandrosterone (DHEA, an endogenous steroid hormone precursor) which has been shown in some studies to have positive effects on bones, cognition, the urogenital tract and libido.22
Outcome: improvement in vasomotor symptoms and migraines
Michelle opts to use a transdermal oestradiol patch which is changed twice weekly (initially 25 μg/24 hours for the first 4 weeks to try and minimise the exacerbation of migraines, increasing to 50 μg/24 hours) along with her LNG-IUS 52 mg. Her vasomotor symptoms and migraines improve within 6 weeks. Tools such as the Menopause Rating Scale23 may assist in the subjective monitoring of HRT on the woman’s quality of life. Michelle should be reminded to have her LNG-IUS 52 mg changed every 5 years while she takes oestrogen replacement therapy or switch to a combined regimen to ensure ongoing endometrial protection.4 She can also be reassured that she will experience very little additional bleeding when transdermal oestrogen replacement therapy is commenced.24
Treatment should initially be reviewed after 3 months to assess efficacy and tolerability, then annually (unless there are other indications for review) to monitor weight, blood pressure and review her medical history. The consultation is also an opportunity to remind Michelle that cervical screening will be every 5 years after the age of 50 and she will be invited for her first mammogram in the next year. Michelle enquires about how long she can remain on HRT. It is explained that there are no arbitrary limits placed on the duration of HRT usage. The decision to continue or discontinue HRT should be made jointly by an informed woman with her healthcare professional.
Vision for menopause care
Prioritising women’s health and well-being is at the forefront of the British Menopause Society’s ‘Vision for Menopause Care’.25 We want women to have accessible, accurate, up-to-date information about the menopause and healthcare professionals (HCPs) with ‘a basic understanding of the menopause and knowledge about where to signpost women for advice, support and treatment’. Ideally, every primary care team should have a HCP with a special interest in this area and effective pathways to specialist menopause services for the management of more complex patients. Where achieved, this would enable women like Michelle to feel empowered to seek menopausal support from a variety of well-informed HCPs across a range of services and to make treatment choices that are tailored to her needs.
Ethics statements
Patient consent for publication
References
Footnotes
Editor's note The details of this case are fictitious. Any resemblance to actual persons, living or dead, or actual events is coincidental.
Contributors KG wrote the paper. DM helped revise the paper.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Commissioned; externally peer reviewed.