Article Text
Abstract
Objectives Combined hormonal contraceptives (CHCs) are the most widely prescribed contraceptive methods in the UK; however, their use is associated with significant cardiovascular risk for women with some medical conditions and risk factors. The objective of this study was to assess the potential change in CHC prescribing among higher-risk women following publication of the UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) in 2006.
Methods A cross-sectional study was conducted using the General Practice Research Database to analyse UK women aged 15–49 years who were prescribed CHCs during the period 2004–2010. Of women prescribed CHCs, those at higher risk of cardiovascular events (with UKMEC Category 3 or 4 risk factors) were identified. The percentage of higher-risk CHC users, among all CHC users, in 2005 (pre-UKMEC) was compared to that in 2010 (post-UKMEC).
Results The percentage of higher-risk CHC users significantly decreased by 0.8% (95% CI 0.68% to 1.02%) following publication of UKMEC [8.1% (95% CI 7.98% to 8.22%) in 2005 vs 7.3% (95% CI 7.14% to 7.38%) in 2010; p<0.001]. However, an estimated 1 74 472 women in the UK were prescribed CHCs in 2010 despite having Category 3 or 4 risk factors. The most common Category 3 or 4 risk factors were body mass index ≥35 kg/m2, hypertension and smoking in women aged ≥35 years.
Conclusions Despite the observed reduction in prescribing of CHCs to higher-risk women after publication of UKMEC, a large number of women with Category 3 or 4 risk factors are still prescribed CHCs. The increased risk of cardiovascular events is unnecessary for many of these women given the availability of alternative contraceptive methods.
- hormonal contraception
- oral contraceptives
- family planning service provision
- cardiovascular diseases
- obesity
- smoking
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/3.0/ and http://creativecommons.org/licenses/by-nc/3.0/legalcode
Statistics from Altmetric.com
- hormonal contraception
- oral contraceptives
- family planning service provision
- cardiovascular diseases
- obesity
- smoking
Key message points
-
Combined hormonal contraceptives (CHCs) are effective and appropriate contraceptive methods for many women but are associated with cardiovascular risks for those with certain medical conditions and risk factors.
-
Since publication of the UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) in 2006, a statistically significant reduction in the prescribing of CHCs to women with Category 3 or 4 risk factors has occurred.
-
However, the number of women still prescribed CHCs despite having Category 3 or 4 risk factors in the UK remains high and could be lowered given the availability of alternative contraceptive methods.
Introduction
Combined hormonal contraceptives (CHCs), which contain both estrogen and progestogen, are the most widely prescribed contraceptive methods in the UK, accounting for 62% of all prescribed contraceptive use.1 Currently available CHC methods include the combined oral contraceptive pill, the transdermal patch and the vaginal ring. Most women are able to use CHCs without a significant health risk; however, these contraceptive methods are not suitable for all women. CHCs are associated with an increased risk of cardiovascular events, even among healthy women,2–7 and some personal characteristics and medical conditions are associated with a further increase in this cardiovascular risk, in some cases to an unacceptable level.
Risk of venous thromboembolism (VTE) has been estimated in a number of studies to increase by two- to six-fold in women using CHCs, compared to non-users.2–4 8–12 The increased risk of VTE for healthy women using CHCs is of clinical relevance; however, it is considerably less than the risk that occurs during pregnancy.12 VTE risk among oral contraceptive (OC) users has consistently been shown to increase further in women with a high body mass index (BMI).10 ,11 ,13 ,14 One study estimated the risk of VTE to be nearly 24-fold higher in OC users with BMI ≥30 kg/m2 than in non-users with BMI <25 mg/m2.14 An association between smoking and risk of VTE among CHC users has also been demonstrated, with one study estimating a two-fold increase in VTE risk in CHC users who smoked compared to non-smokers.13
The use of CHCs has also been associated with a two- to three-fold increase in ischaemic stroke risk,5 ,6 which has been estimated to increase further, to approximately 11-fold, among women with a history of hypertension.6 Migraine has also been shown to be an independent risk factor for ischaemic stroke and, although limited evidence is available, synergism between CHC use and migraine for stroke risk has been reported.15 ,16 OC use has been estimated to confer a 2.5-fold increase in risk of myocardial infarction (MI), compared to women who have never used OCs.7 The risk of MI was shown to increase further in OC users who smoked, had hypertension or had hypercholesterolaemia, with odds ratios (OR) of 9.52 [95% confidence interval (CI) 5.41–16.72], 9.30 (95% CI 3.89–22.23) and 9.90 (95% CI 1.83–53.53), respectively, compared to non-users who did not have the risk factor.7
The UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) were first implemented in 2006 and provide evidence-based recommendations for safe provision of contraception to women with a range of medical conditions and personal characteristics.17 The UKMEC were adapted from the MEC published by the World Health Organization (WHO) in 200418 to reflect UK clinical practice. An updated version of UKMEC was subsequently published in 2009.19 The UKMEC classifies conditions in one of four categories, depending on their associated risk for women using contraception, as defined in Table 1. Risk factors for CHC use classified as Category 3 or 4 by UKMEC include obesity (BMI ≥35 kg/m2), hypertension, current or past year smoking in women aged ≥35 years, migraine, family history of VTE (first-degree relative aged <45 years), personal history of or current VTE, history of or current ischaemic heart disease, and history of stroke.17 ,19 Despite publication of the guidance there is concern that awareness and implementation of UKMEC among general practitioners (GPs) is low and that a proportion of women using CHCs in the UK remain at an unnecessary risk of cardiovascular events.
UK Medical Eligibility Criteria for Contraceptive Use (UKMEC) definitions for categories of risk factors for contraceptive use19
The General Practice Research Database (GPRD) is a computerised database containing anonymised medical records from primary care. It is the largest database of this type in the world and currently data are being collected for approximately 5.2 million active patients in the UK.20 The GPRD provides data on primary care prescribing as well as patient characteristics and medical conditions for a representative sample of the UK population. The GPRD was replaced by the Clinical Practice Research Datalink in March 2012.21
The objective of the current study was to estimate the proportion of women prescribed CHCs who had Category 3 or 4 risk factors before and after the 2006 publication of UKMEC, and to assess whether a change in prescribing of CHCs occurred following UKMEC publication. Category 3 or 4 risk factors are defined as usually outweighing the advantages of CHCs or representing an unacceptable health risk, respectively, and for the purposes of this study they represent women at higher risk of cardiovascular events.
Methods
This was a cross-sectional study of women registered on the GPRD between 1 January 2004 and 31 December 2010. The GPRD contains longitudinal clinical data about patients recorded by GPs since 1987 and is representative of UK general practice. The data include patient socio-demographic characteristics, details of GP visits, diagnoses from specialists, referrals and hospital admissions, prescriptions, and results of laboratory tests.20 ,22 The data are systematically recorded and sent anonymously to the Medicines and Healthcare products Regulatory Agency, which collects and organises this information. The GPRD has been validated for use, and is extensively used, in outcomes research.
Data were extracted from the GPRD for each calendar year (January–December) during the 2004–2010 study period for women aged 15–49 years (inclusive), prescribed at least one prescription for a CHC, who had at least one UKMEC Category 3 or 4 risk factor during that calendar year. CHCs were included in the study if listed in the Monthly Index of Medical Specialities (MIMs) (December 2010): the combined patch (ethinylestradiol+norelgestromin), combined pills (ethinylestradiol+desogestrel, ethinylestradiol+drospirenone, ethinylestradiol+gestodene, ethinylestradiol+levonorgestrel, ethinylestradiol+norethisterone, ethinylestradiol+norgestimate, mestranol+norethisterone), combined vaginal ring (ethinylestradiol+etonogestrel) and phasic combined pills [estradiol+dienogest (phasic), ethinylestradiol+gestodene (phasic), ethinylestradiol+levonorgestrel (phasic), ethinylestradiol+norethisterone (phasic)].
The 2006 and 2009 UKMEC publications were used to select the Category 3 or 4 risk factors most relevant to UK clinical practice, which were examined in the study.17 ,19 Some changes were made to the categorisation of risk factors between the 2006 and 2009 versions of UKMEC; however, there was no change to the risk factors classified as either Category 3 or 4, so the risk factors included in our study did not change. Category 3 and 4 risk factors have been grouped for the purposes of this study; however, it is important to note that the definitions and recommendations for prescribing CHCs to women with these risk factors differ between the two groups (Table 1).
The data available in the GPRD were not sufficient to provide accurate information about every aspect of some of the Category 3 or 4 risk factors selected from UKMEC, therefore only the available data were extracted for these risk factors. For example, although UKMEC specifies several Category 3 or 4 risk factors related to VTE, only data for current VTE or personal history of VTE could be extracted from the database and included in the study.
The following ten UKMEC Category 3 or 4 risk factors were examined in the study:
-
Current smokers aged 35 years or over
-
Past year smokers aged 35 years or over (i.e. stopped smoking within the last year)
-
BMI ≥35 kg/m2 (if not available, BMI was calculated using weight and height)
-
CHC prescribed within 21 days of childbirth
-
Hypertension
-
Personal VTE (current and history of VTE)
-
Ischaemic heart disease (IHD) (current and history of IHD)
-
History of stroke
-
Dyslipidaemia (restricted to people with known familial hyperlipidaemias, total cholesterol >5 mmol/dl and prescribed statins, fibrates, niacin or ezetimibe)
-
Migraine with aura.
This study was designed to compare CHC prescribing before and after UKMEC publication, therefore the study period was chosen by examining data either side of the date of the first UKMEC publication (June 2006). However, the Quality and Outcomes Framework (QOF), which is a voluntary incentive scheme for GPs in the UK, was introduced in April 2004. Initially data were studied from 2003; however, as the introduction of QOF may have impacted the quantity and quality of recording in the GPRD, the period prior to QOF introduction was excluded from the analysis, resulting in a study period from January 2004 to December 2010.
The study data were extracted from the GPRD on a yearly basis for the 2004–2010 period, using the aforementioned list of risk factors as search terms, as well as commonly used synonyms for each condition. Data extraction was conducted in October 2011 using data available up to 31 December 2010. The total number of women aged 15–49 years, the number of CHC users and the number of higher-risk CHC users with at least one UKMEC Category 3 or 4 risk factor in this age group were extracted from the database. This allowed calculation of the proportion of CHC users and higher-risk CHC users out of the women registered on the GPRD for each calendar year studied. These proportions were then used to estimate the number of higher- risk CHC users in the UK, based on UK population data for each year. Data were also analysed by individual risk factor and by age group.
The statistical analyses were performed using SAS Version 9.1 software (SAS Institute Inc., Cary, NC, USA). Statistical tests were conducted comparing 2005 to 2010 as these were, respectively, the closest year prior to UKMEC publication in 2006 and the most recent year for which data were available. Assuming independence between the years 2005 and 2010, a t-test was used to evaluate whether a statistically significant difference existed in the proportion of higher-risk CHC users in 2005 compared to 2010. As women could have been part of both the 2005 and 2010 cohorts, the generalised estimated equation (GEE), with patient ID as the repeated measurement, was used to account for the violation of the independence assumption in the t-test. A Chi-square (χ2) test was also performed to test whether there was an association between the independent variables of year (2005 and 2010) and the percentage of women with Category 3 or 4 risk factors.
Results
The number of women aged 15–49 years registered on the GPRD increased by 8.3% between 2004 and 2010, ranging from 1 018 835 to 1 103 669 women, respectively (Table 2). In contrast, over the same period the number of GPRD-registered CHC users aged 15–49 years decreased by 9.8% from 199 105 in 2004 to 179 558 in 2010.
Number and percentage of combined hormonal contraceptive (CHC) users and higher-risk CHC users in the General Practice Research Database13
The number of higher-risk CHC users (with a Category 3 or 4 risk factor) registered on the GPRD was 16 121 in 2004 (8.1% of all CHC users, 95% CI 7.98% to 8.22%), and this decreased to 13 028 women (7.3% of all CHC users, 95% CI 7.14% to 7.38%) in 2010 (Table 2, Figure 1). The absolute reduction in the percentage of higher-risk CHC users from 2005 to 2010 was 0.8% (95% CI 0.68% to 1.02%) and both the t-test and χ2 test demonstrated that this reduction was statistically significant. The t-test showed a statistically significant difference (p<0.001) between the proportion of higher-risk women in 2010 compared to 2005, when no other factors were controlled. The GEE estimation, which was applied to address the violation of the independence assumption in the t-test, showed that the two cohorts are independent (p<0.001). The χ2 test showed a statistically significant association (p<0.001) between the year (2005 and 2010) and the percentage of women with Category 3 or 4 risk factors, when no other factors were controlled.
Percentage of combined hormonal contraceptive (CHC) users with Category 3 or 4 risk factors among all CHC users aged 15–49 years in the General Practice Research Database.
Based on the proportion of higher-risk CHC users in the GPRD (8.1% in 2004 and 7.3% in 2010) it is estimated that the number of women using a CHC with a Category 3 or 4 risk factor in the whole UK population was 227 985 in 2004 and 174 472 in 2010. This was calculated based on a population of women aged 15–49 years in the UK of 14 408 500 in 2004 and 14 708 400 in 2010,23 of whom 19.6% and 16.3% were assumed to be CHC users, respectively.
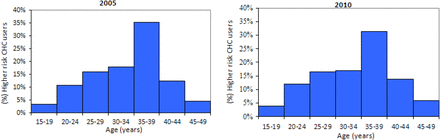
The distribution of the ages of higher-risk CHC users was similar pre- and post-publication of UKMEC (Figure 2). Some 52.2% and 51.0% of CHC users with Category 3 or 4 risk factors were aged ≥35 years in 2005 and 2010, respectively; however, it must be noted that two of the risk factors studied specifically identified women in this age group (current smokers and past year smokers aged ≥35 years).
Distribution of higher-risk combined hormonal contraceptive (CHC) users by age group.
In 2005, prior to publication of UKMEC, hypertension was the most common Category 3 or 4 risk factor, accounting for 34.2% of all higher-risk CHC users (Figure 3). This was followed by BMI ≥35 kg/m2 (31.2% of all higher-risk users), past year smoking in women aged ≥35 years (21.1%) and current smoking in women aged ≥35 years (20.0%). In 2010, these same four risk factors were still considerably more common among CHC users than other factors, but BMI ≥35 kg/m2 was the most commonly recorded Category 3 or 4 risk factor (35.3% of higher-risk users), followed by hypertension (32.7%) and past and current smoking in women aged ≥35 years (17.6 and 17.7%, respectively).
Percentage of higher-risk combined hormonal contraceptive (CHC) users with each specific risk factor studied (2005 and 2010). BMI, body mass index; CS, current smoker; IHD, ischaemic heart disease; PS, past year smoker; VTE, venous thromboembolism.
Discussion
This study shows that a small but significant decrease has occurred in the percentage of women prescribed CHCs with Category 3 or 4 risk factors since the publication of UKMEC in 2006 [8.1% (95% CI 7.98% to 8.22%) in 2005 vs 7.3% (7.14% to 7.38%) in 2010; p<0.001]. The results demonstrate a temporal association between the publication of UKMEC and the decrease in the proportion of higher-risk women prescribed CHCs; however, this study cannot confirm that a causal relationship exists. Despite finding a statistically significant reduction, the absolute difference between the percentages of higher-risk CHC users in 2005 and 2010 is small (0.8%) and may not be clinically meaningful. The observed significant statistical difference may be due to the large sample size.
In 2010, an estimated 174 472 women in the UK were still prescribed CHCs even though they had a Category 3 or 4 risk factor. This highlights that a high number of women in the UK are still receiving CHCs, despite having a risk of cardiovascular events which is defined by UKMEC as usually outweighing the advantages of using CHCs or representing an unacceptable health risk. Stronger implementation measures could improve clinicians’ awareness of the UKMEC recommendations and contribute to a lower number of women with Category 3 or 4 risk factors being prescribed CHCs. An electronic alert system could be implemented, whereby the prescriber is informed of the UKMEC recommendations when prescribing a CHC to a woman with a recorded risk factor.
The increased health risk faced by these women is unnecessary for many given the availability of alternative contraceptive methods, which have lower associated cardiovascular risk. Options for women who should not receive CHCs include progestogen-only methods, such as progestogen-only pills (POPs) and progestogen-only long-acting reversible contraception (LARC) methods, as well as intrauterine devices (IUDs). A WHO case-control study demonstrated no significant increase in cardiovascular risk (including stroke, VTE and acute MI) among women receiving POPs and progestogen-only injectables,24 and a recent cohort study also demonstrated that POPs and IUDs were not associated with increased risk of venous thrombosis.2
The most common Category 3 or 4 risk factors found among CHC users in the UK were high BMI (≥35kg/m2), hypertension and current or past year smoking in women aged ≥35 years. Of these common risk factors, the percentage of women with high BMI increased between 2005 and 2010 and the percentage of current and past year smokers aged ≥35 years decreased over the same time period (Figure 3). These changes over the study period are likely to be due to changes in the numbers of women with these risk factors in the UK population over this time interval. UK data suggest that obesity rates increased by 13% and smoking rates among women of all ages declined by 25% during the study time frame.25 This highlights the challenges currently faced in UK clinical practice where obesity and related morbidities are becoming increasingly common, creating the need for additional consideration when prescribing contraception.
Although UKMEC Category 3 and 4 risk factors have been combined for the purposes of this study, the different recommendations for prescribing CHCs to these two groups should be noted. For women with Category 3 risk factors CHCs are not usually recommended, unless other more appropriate methods are not available or not acceptable. Among women with Category 4 risk factors the condition is defined as representing an unacceptable health risk if the CHC is used (Table 1).
The observed decrease in the percentage of higher-risk women prescribed CHCs may have been caused by factors other than the implementation of UKMEC, such as the increase in use of progestogen-only LARC following publication of the National Institute for Health and Clinical Excellence LARC guideline in 2005.26 This is supported by the observation that the overall percentage of women who received CHCs decreased from 19.6% in 2004 to 16.3% in 2010. The decreased use of CHCs both in the whole population and among higher-risk women could also be a reflection of inaccurate recording of information in the GPRD.
We were unable to identify any other published studies that have assessed the impact of UKMEC implementation on CHC prescribing. A European survey assessing CHC prescribing following implementation of the WHO MEC estimated that 12% of women receiving prescribed contraceptives who had conditions for which estrogen-containing methods were not preferred were still receiving a COC.27
A limitation of the present study, as with all database studies, is that it relied on accurate and complete recording in the database. It is possible that in some cases conditions were recorded in the GPRD prior to an alternative final diagnosis, therefore an incorrect diagnosis was recorded and extracted. In addition, results for the risk factor of childbirth within 21 days may not be reliable given the short time window for recording this information. Also, women may be provided with the pill within 21 days of childbirth and advised to delay starting it until after Day 21. The impact of these limitations is expected to be the same each year, and therefore should not affect the comparison between before and after the publication of UKMEC.
Other limitations of the study include inadequate detail in the GPRD meaning that not all Category 3 or 4 risk factors could be assessed; the study does not take into account the publication of the updated version of UKMEC in 2009; and the t-test assumes independence between the two groups of women in 2005 and in 2010. In addition, the potential impact of gradual implementation of QOF indicators from 2004 onwards on recording in GP databases was not considered.
It would be useful to repeat this study in several years’ time to determine whether the benefit observed following UKMEC publication continues, and to specifically assess whether there has been an improvement in the prescribing of CHCs to women with high BMI. In addition, it would be interesting to investigate the impact of the publication of the 2009 UKMEC.
In conclusion, publication of the UKMEC appears to have made a small, positive impact on the proportion of higher-risk women prescribed CHCs. However, in 2010 an estimated 174 472 women in the UK (7.3% of CHC users) were still prescribed CHCs despite having recorded Category 3 or 4 risk factors. Many of these women were at an unnecessarily increased risk of cardiovascular events, given the availability of alternative contraceptive methods with lower associated cardiovascular risks. In 2010, the most common Category 3 or 4 risk factor observed among women receiving CHCs was high BMI, which reflects the challenges that the increasing obesity prevalence poses in UK clinical practice when prescribing contraceptives. Further implementation of the UKMEC is required to reduce the large number of higher-risk women who are still receiving CHCs in the UK.
References
Footnotes
-
Funding This study was funded by Merck Sharp & Dohme Ltd.
-
Competing interests Three of the authors are employed by Merck Sharp and Dohme Ltd or Merck & Co., Inc.
-
Ethics approval Independent Scientific Advisory Committee for MHRA database research (ISAC); protocol number 09_127RA.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue