Article Text
Statistics from Altmetric.com
- etonogestrel implants
- hydro-dissection
- ultrasound guided procedure
- unsuitable for removal with standard techniques
- implants
Background
In 2011 Merck Sharp & Dohme (MSD) introduced a new etonogestrel (ENG) implant, Nexplanon, with a different type of insertion technique from its predecessor, Implanon. Over subsequent years it has become clear that the problem of incorrect placement of ENG implants, which are then unsuitable for removal using standard techniques, has continued. An effective and safe technique is needed to remove such implants, particularly for women wishing to conceive.
The correct plane of insertion is 1–2 mm below the skin surface, where the implant is readily palpable and can usually be removed using the ’pop-out' technique. When insertion is deep to the subdermal layer, implants become more difficult to palpate and remove. However the term ’deep' is too limited to explain the range of depths and different degrees of difficulty encountered with removal. An implant that is deep to the subdermal layer but superficial to the biceps or triceps fascia will still usually be palpable, although where there is uncertainty of the position, then only once the exact site is clarified with ultrasound may the ability to feel it with deep palpation be appreciated.1 Most ’deep' implants that lie above muscle fascia do not need a ’needle lift' procedure as they can easily be removed with the simpler ’U' technique using ring forceps. However, once an implant is within or below the fascia it will become impalpable, even in thin women. These fascial or subfascial implants are unsuitable for removal using the standard techniques mentioned above, so most removers who are able to do so will then choose an ’open' approach.2
Why is a different approach needed?
In cases where the implant lies immediately adjacent to neurovascular structures many practitioners will decline to perform removal owing to fears that the open dissection could result in damage to these structures. Here I describe a simple alternative method for removal of a precariously sited contraceptive implant.
Does the implant need to be removed?
The main indication for implant removal is for women wanting to conceive. Where there is no intention of future pregnancy and the implant is precariously sited, then the option of leaving the implant in place should be considered to avoid risk of damage to vulnerable structures. There is no absolute reason to remove an implant because it is time-expired. If an implant remains in situ, ENG is detectable in the serum for many years and it may have a contraceptive action through endometrial suppression well beyond its licensed duration (author’s verbal communication with MSD). However, there are no reports to date of the long-term presence of an implant adversely affecting health.
Case report
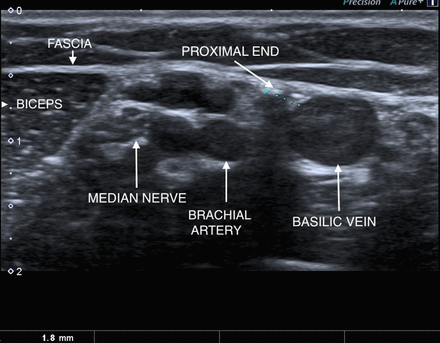
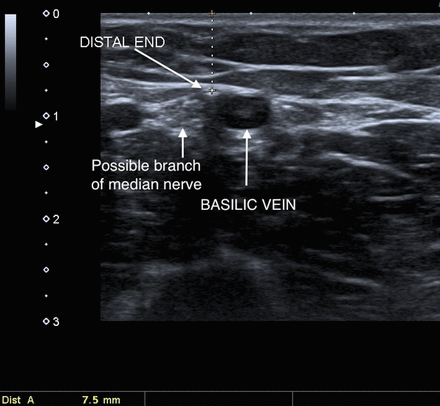
A 22-year-old woman was referred requesting removal of a non-palpable implant in her right arm. She had previously seen two other specialist ‘deep implant’ removers, and they considered that it was unsuitable for removal with standard techniques (pop-out or U technique). They both declined to offer an ’open' technique owing to the very close proximity to neurovascular structures. The implant was completely non-palpable, but was easily located with ultrasound at around 7.5 mm depth in the groove between biceps and triceps, approximately 8–10 cm above the medial epicondyle. The proximal end of the implant was 1.8 mm lateral to the basilic vein (figure 1). The distal end of the implant was also less than 2 mm from the vein, but also there was a structure resembling a nerve immediately medial and inferior to the implant (figure 2). The median and ulnar nerves where seen separately and more medial to this so the possibility the median nerve was branching was considered. Reassuringly at no time had the woman experienced abnormal sensation, and applying pressure over the implant did not induce any pain or paraesthesia. The brachial artery and ‘main’ median nerve could both be viewed around 5 mm deeper and medial to the implant. At the proximal end of the implant several other vessels ran in close proximity, illustrating the anatomical variability observed at this site (figure 1).
Cross-section of the right inner arm. Transducer applied transversely to long axis at mid-arm level. Anatomical relations at proximal end of the implant are demonstrated.
Sliding transducer down arm to distal end of the implant shows it slightly closer to the basilic vein at this level and also demonstrates a nearby nerve-like structure.
The position of the implant was explained to the patient and she was clear about her decision for removal. In view of its very close proximity to the vein and possibly a branch of the median nerve, there was discussion of the option of attempting to move the implant to a safer location for removal using ultrasound-guided injection of local anaesthetic. The consent form documented the possible risk of damage to nerves or blood vessels and possible failure to remove the implant.
What procedure was used?
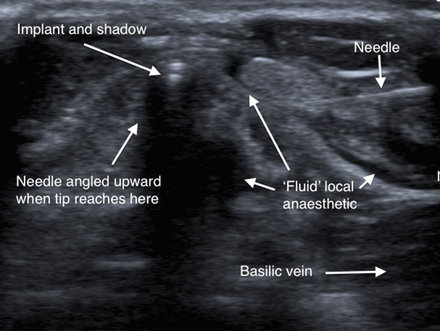
With the arm supported on a pillow in a suitable position for the removal, the implant position was marked. The skin was cleansed with chlorhexidine 20% and a sterile field was then created using paper drapes. The linear ultrasound transducer was cleaned and placed in a sterile transducer bag along with sterile gel on the footprint inside and outside the bag. The skin was infiltrated with 0.5 mL 2% lidocaine at two points approximately 1.5 cm medial and 1.5 cm lateral to the mid-shaft of the implant. A standard 21-gauge green needle on a 5 mL syringe containing further local anaesthetic was then used to puncture the skin at the anaesthetised medial site and inserted 0.5–1 cm towards the implant. The transducer was then applied to the skin over the implant and the needle readily visualised approximately 1 cm lateral and superficial to the implant. The anatomical structures and the implant were viewed in short axis (cross-section) while the needle was viewed in long axis. Keeping the needle tip in view at all times, very small amounts of local anaesthetic, around 3 mL in total, were injected as the needle tip was carefully advanced down and towards the implant (figure 3). As fluid was injected it could be seen to separate the tissue planes, and as the needle tip got closer and deep to the implant, the implant could be seen to readily move up and away from the injected fluid. This created a fluid-distended space between the implant and the vein and also moved the implant away from all the neurovascular structures. The needle could then readily be advanced under the implant and angled upward to underpin it, with the needle tip exiting the skin through the lateral anaesthetised site (figure 4), where the needle tip is secured and protected by applying a small artery forceps. It was then very simple to make a 3 mm puncture in the skin over the implant with a size 11 scalpel blade and using gentle stroking movements incise down to ’feel' the elevated implant with the scalpel tip. A ring forceps was introduced through the puncture and the implant grasped and removed, as in the U technique. Once the implant was grasped in the ring of the forceps the needle was removed. The puncture was closed with Steristrips.
Injected local anaesthetic can be seen to separate tissue planes. The needle has elevated the implant and overlying fascia away from the vein and deeper anatomical structures.
The implant is now 4.4 mm deep to the surface whereas previously it was 7.5 mm deep. It is safely distant from the vein, artery and nerve, which cannot now be visualised owing to the needle’s acoustic shadow.
Is this modification in practice beneficial?
Ultrasound permits excellent visualisation of the implant and surrounding structures.
Anatomy is very variable, as exemplified here, with at least five vessels visible in the groove between biceps and triceps. With this technique these vessels are visualised constantly as the needle is advanced.
The risk of damage to nerves and blood vessels is minimised by moving the implant away from these structures using fluid under direct vision. This should carry much less risk of trauma than use of retractors and multiple instruments through an open incision.
There is no risk of mistaking a ’white' nerve trunk for the white implant. They may appear very similar when exposed during open dissection but they are distinctly different on ultrasound.
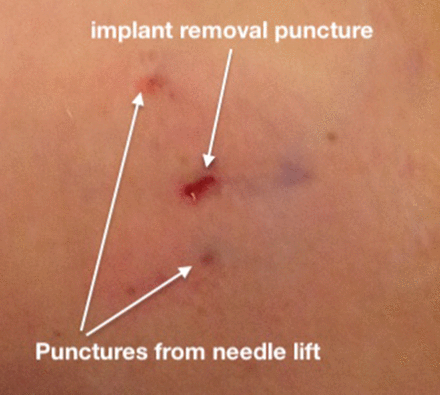
A small (minimal access) puncture (figure 5) does not require closure of the fascia, carries a low risk of infection or poor healing, and produces less scarring than an open incision.
The ultrasound evaluation, determining the best approach, positioning the arm and marking it takes a number of minutes. However completing the procedure then is minimally invasive and very rapid (less than 5 min).
Example of a removal puncture following subfascial implant removal using the needle lift technique.
Is hydrodissection a new technique?
Hydrodissection as a safe method of bluntly and non-traumatically dissecting tissue planes is a proven, time-tested surgical technique. It has established a place in foreign body removal,3–6 to preserve nerve function in nerve entrapment syndromes,7 8 to preserve blood vessels in surgical reconstruction, and to establish surgical planes within the eye in ophthalmic surgery.9
What advice is there for training in ultrasound-guided procedures?
The key to safe removal of deeply sited and non-palpable implants is ultrasound evaluation of the position and proximity to neurovascular structures. Using the correct equipment, a high-frequency linear transducer, it is relatively easy to learn to recognise these structures, and also to recognise the characteristic features of an implant. Ultrasound-guided needle insertion can best be taught using a phantom containing simulated nerves, blood vessels and an adjacent implant, which can all be visualised with ultrasound. The success and safety of the procedure requires needle and transducer control with constant visualisation of the needle tip, and a clear understanding of the local ultrasound anatomy. When ability has been demonstrated on a phantom, the trainee could move on to supervised procedures, initially removing implants within muscle. Cases of ‘deep’ location within muscle are much more common than cases where the implant lies adjacent to vulnerable neurovascular structures, and they are very suitable for the needle lift technique. The procedure is simpler as there is no need to use hydrodissection since vital structures are not in such close proximity. However, it is appropriate to inject local anaesthetic as the needle is advanced, as pain sensation returns as one penetrates the fascia. So in these cases the trainee can observe how injecting small amounts of fluid readily moves the implant relative to anatomical structures, before progressing to cases requiring hydrodissection.
Acknowledgments
My thanks to thank Stephen Searle who converted me (the sole author) from the open to the needle lift technique. Observing the readiness with which implants deep to fascia would move during the needle lift technique led me to realise that hydrodissection could be used to apply this technique where an implant is precariously close to a vulnerable anatomical structure.
Footnotes
Contributors MP is the sole author of the paper.
Funding This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement None of the material has been submitted or published elsewhere.
Linked Articles
- Highlights from this issue