Article Text
Abstract
Background The contraceptive efficacy and tolerability of a new flexible extended regimen of ethinylestradiol (EE) 20 μg/drospirenone (DRSP) 3 mg to extend the menstrual cycle and enable management of intracyclic (breakthrough) bleeding (flexibleMIB) was investigated and the bleeding pattern compared with a conventional 28-day regimen and a fixed extended 124-day regimen.
Study design This Phase III, 2-year, multicentre, open-label study randomly (4:1:1) allocated women (aged 18–35 years) to the following regimens: flexibleMIB (24–120 days' active hormonal intake with 4-day tablet-free intervals); conventional (24 days' active hormonal intake followed by a 4-day hormone-free interval); or fixed extended (120 days' uninterrupted active hormonal intake followed by a 4-day tablet-free interval). Primary outcomes included the number of bleeding/spotting days during Year 1 (all regimens) and the number of observed unintended pregnancies over 2 years (flexibleMIB only).
Results Results were analysed in 1067 women (full analysis set). The mean number of bleeding/spotting days was lower with the flexibleMIB vs the conventional regimen [41.0±29.1 (95% CI 38.8–43.3) vs 65.8±27.0 (95% CI 62.2–69.4) days, p<0.0001; treatment difference −24.8 (95% CI −29.2 to −20.3) days]. The corresponding value for the fixed extended regimen was 60.9±51.1 (95% CI 53.9–67.9) days. The Pearl Index for the flexibleMIB regimen was 0.64 (95% CI 0.28–1.26). All regimens had comparable tolerability profiles.
Conclusions EE 20 μg/DRSP 3 mg administered as a flexible extended regimen with MIB is effective, well tolerated and is associated with statistically significantly fewer bleeding/spotting days and fewer withdrawal bleeding episodes vs EE/DRSP in a conventional 28-day regimen. The flexibleMIB also provided statistically significantly fewer spotting days vs EE/DRSP in a fixed extended 124-day regimen (post hoc evaluation). The flexibleMIB regimen allows women to extend their menstrual cycle and manage their intracyclic (breakthrough) bleeding.
This paper is freely available online under the BMJ Journals unlocked scheme, see http://jfp.bmj.com/info/unlocked.dtl
Statistics from Altmetric.com
Introduction
Many modern-day combined oral contraceptives (COCs) have a 28-day cycle, usually comprising 21 days of active (i.e. hormonal) tablets followed by a 7-day hormone-free interval. Newer COCs with a shortened hormone-free interval (i.e. 24 days of active hormonal intake followed by a 4-day hormone-free interval), which are associated with more sustained ovarian suppression and reduced hormonal fluctuations,1 are available; such COCs still, however, follow the 28-day cycle paradigm. The 28-day cycle was originally designed to allow for monthly bleeding, even though this was not necessary, primarily to mimic the natural menstrual cycle and to reassure women that they were not pregnant.2
Key message points
▶ A flexible extended regimen of ethinylestradiol (EE) 20 μg/drospirenone (DRSP) 3 mg is an effective and well-tolerated combined oral contraceptive.
▶ A flexible extended regimen of EE/DRSP is associated with fewer bleeding/spotting days than a conventional 28-day regimen and a fixed extended 124-day regimen of EE/DRSP.
▶ The flexible extended regimen of EE/DRSP allows women to extend their menstrual cycle and manage any intracyclic (breakthrough) bleeding that may occur during the flexible intake phase.
The long-term availability of COCs with a hormone-free interval has led many women to mistakenly believe that it is natural to have a withdrawal bleed during COC use or that missing a withdrawal bleed is associated with negative health effects.3,–,7 Despite this, given the choice, a substantial proportion of women would welcome a reduction in the frequency of their menstrual bleeding. Survey data indicate that as many as 59% of women aged 15–49 years would be interested in not menstruating every month.7 8 Furthermore, up to 46% of such women would rather not have a period at all.7 8 Reasons for wanting to reduce menstrual bleeding include reducing monthly menstrual-related complaints, better hygiene and improved quality of life.5 7
At present, women who are prepared to use COCs and want to reduce the frequency of their menstrual bleeding have two options. First, they can opt to (either occasionally or regularly) skip the hormone-free interval of their cyclical COC, thus extending the length of time that they take active treatment;2 although continually postponing the use of conventional regimen COCs is not a labelled indication. Second, they can opt to use COCs in fixed extended regimens of up to 1 year;9,–,12 however, these preparations are currently only available in the USA. Fixed extended regimens have been shown to be effective with safety profiles that are similar to conventional COCs;5 9 10 12,–,14 however, they are often associated with an increased risk of breakthrough bleeding versus cyclical COCs.12 14 Many women are not willing to tolerate such bleeding, and in some studies overall dropout rates of greater than 50% have been observed.5 9 10 12 14
Ethinylestradiol (EE) 20 μg/drospirenone (DRSP) 3 mg in a 24/4 regimen is an effective and well-tolerated COC.15,–,17 It was hypothesised that a regimen that extended beyond the conventional 28 days and gave women the flexibility to manage their intracyclic (breakthrough) bleeding (MIB) would be appealing. The objective of the current study was to investigate the efficacy and tolerability of EE 20 μg/DRSP 3 mg administered in a flexible extended regimen (‘flexibleMIB’) that allowed women to extend the menstrual cycle as well as manage their intracyclic bleeding in comparison to a conventional 28-day regimen. A fixed extended 124-day regimen was also included to assess the safety over a continuous 120-day intake period, the maximum duration possible with the flexibleMIB regimen.
Methods
Study design
This was a Phase III, multicentre, randomised, open-label, parallel-group study conducted at 37 centres in Germany, Canada and The Netherlands between December 2005 (first subject, first visit) and October 2008 (last subject, last visit). The study (protocol number, 308683; ClinicalTrials.gov identifier, NCT00266032) was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation–Good Clinical Practice guidelines. All participants received adequate information about the study and signed an informed consent form before entering the study.
The study consisted of two phases: a 1-year comparative phase where women were randomly allocated to receive EE 20 µg/DRSP 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen and a 1-year safety extension phase during which the majority of women received the flexibleMIB regimen. For the most part, data from the 1-year comparative phase are reported here, while data from the safety extension phase are reported elsewhere.18
Study population
Healthy females aged between 18 and 35 years who requested contraceptive protection were eligible to participate in the study. Women were required to be in good general health with a normal cervical smear test result at or in the 6-month period prior to screening, and smokers could participate so long as they were 30 years of age or younger at the time of screening. The main exclusion criteria included: use of other contraceptive methods, sterilisation, pregnancy or lactating, body mass index <18 and >30 kg/m2, presence or history of any vascular diseases or coagulation disorders, known hypersensitivity to any of the study drug ingredients, and any disease or condition that could interfere with the study medication.
Study regimens
Flexible extended regimen with management of intracyclic (breakthrough) bleeding
With the flexibleMIB regimen, women received EE/DRSP for a flexible number of cycles (minimum three, maximum 13 in 1 year), each of which was separated by a 4-day tablet-free interval. With this regimen, tablet taking was continuous for at least 24 days (‘mandatory phase’); in this manner, the minimum cycle length was 28 days and the design of the COC was based on the approved and marketed conventional 24/4-day regimen of EE 20 µg/DRSP 3 mg (YAZ®; Bayer HealthCare Pharmaceuticals). After the mandatory phase, the cycle could continue up to a maximum of 120 consecutive days (i.e. for a maximum cycle length of 124 days). A 4-day tablet-free interval had to be taken at the latest after 120 days of continuous tablet intake. Between Days 25 and 120 (‘flexible phase’) women were instructed to observe a 4-day tablet-free interval if they experienced three consecutive days of bleeding and/or spotting. After each 4-day tablet-free interval, women started a new cycle with a minimum of 24 days and a maximum of 120 days of tablet intake.
Conventional 28-day regimen
With the conventional 28-day regimen, women received EE/DRSP for 13 cycles. Each cycle comprised 24 days of active hormonal intake followed by a 4-day interval in which women took hormone-free (placebo) tablets to induce withdrawal bleeding.
Fixed extended regimen
With the fixed extended regimen, women received EE/DRSP for three cycles. Each cycle comprised 120 days of uninterrupted active hormonal intake followed by a 4-day tablet-free interval.
Study medication was to be commenced on the first day of menstrual bleeding (new COC starters) or scheduled withdrawal bleeding (COC switchers) following the baseline visit. During active hormonal intake, tablets could be taken in the morning or the evening, but the interval between two tablets had to remain as close as possible to 24 hours. If a woman missed a tablet she was to take it as soon as possible (even if this meant taking two tablets at the same time); thereafter, tablet intake was to continue as normal. With regard to the rules for the use of back-up contraception, a missed tablet was defined as tablet intake that was delayed by more than 24 hours.
Study outcomes
Primary outcomes
The main primary outcome was the total number of bleeding/spotting days during the 1-year comparative phase. The total number of bleeding/spotting days per subject was calculated by summing all of the days with a bleeding intensity of spotting or greater.
The other primary outcome was the number of observed unintended pregnancies with the flexibleMIB regimen over the 2 years of treatment. (This variable was originally a secondary outcome, but was changed to a co-primary outcome based on a recommendation by the Food and Drug Administration during a scientific advisory meeting.) The number of observed unintended pregnancies was used to measure contraceptive efficacy, calculated using the Pearl Index (number of pregnancies divided by the exposure in woman-years multiplied by 100) and by life-table analysis. The Pearl Index was only calculated in women who received the flexibleMIB regimen as the number of women in the other two groups were planned for a comparison of bleeding pattern and safety and were, therefore, not powered for a reliable calculation of the pregnancy rate. A life-table analysis was performed for the time to the occurrence of a pregnancy. The cumulative failure rate (i.e. the probability of getting pregnant) was calculated using the Kaplan–Meier estimator on the basis of pregnancies that were considered to have occurred after up to 2 years of treatment.
Secondary outcomes
Secondary outcomes included bleeding pattern and cycle control outcomes over the first year. Bleeding pattern parameters included the number and length of bleeding/spotting episodes and spotting-only episodes (days with bleeding/spotting or spotting only preceded and followed by at least two bleed-free days); these parameters were determined for each subject for 90-day reference periods. The first reference period started on the first day of study medication intake. Cycle control parameters included withdrawal bleeding, intracyclic bleeding, scheduled bleeding and unscheduled bleeding defined according to the different regimens (Table 1). Cycle control parameters were determined for each subject at every cycle.
Definitions of withdrawal bleeding, intracyclic bleeding and scheduled and unscheduled bleeding in women who received ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, fixed extended or conventional 28-day regimen
Bleeding data were recorded by every subject using diary cards that were to be completed on a daily basis. Each day, subjects rated their bleeding as none, spotting, light, normal or heavy. Diary records were to start with the onset of the first menstrual or withdrawal bleeding episode following the baseline visit (i.e. the bleeding episode that prompted the initiation of study medication), and were to continue until the end of the last bleeding episode within the study. The daily diary cards were also used to monitor compliance with study medication.
A self-reported, non-validated, menstrual bleeding questionnaire was used to assess the impact of the three regimens on subjects' menstrual bleeding and the problems associated with it; data were collected retrospectively for the 12 weeks prior to inclusion into the study and at four time points during the study. Subjects in each of the three groups also completed the 22-item validated Psychological General Well-Being Index (PGWBI) at baseline and at two time points during the study. A third questionnaire was used to assess satisfaction with the regimen in women who received the two extended regimens; this was completed at the end of the first year of the study.
Safety outcomes
The following safety outcomes were evaluated: adverse events (AEs); laboratory variables; bone markers and bone mineral density; general physical and gynaecological findings (including the outcomes of cervical smear tests); endometrial biopsies; and vital signs and body weight. AEs for the first year of the study are reported here; data pertaining to long-term safety outcomes are reported separately.18
Statistical analysis
Descriptive statistics [number, mean, standard deviation (SD)] were calculated for each quantitative variable, while frequencies were given for categorical data. Regarding the primary outcome of the number of bleeding/spotting days, the main evaluation was a comparison of the flexibleMIB regimen versus the conventional regimen using Student's t-test assuming a normal distribution. A statistical comparison between the flexibleMIB and the fixed extended regimen was not planned, although a post hoc analysis was conducted.
For the 1-year comparative phase, subjects were randomly allocated in a 4:1:1 ratio to the flexibleMIB, conventional and fixed extended regimens. The randomisation sequence was generated by the responsible biometrician and performed by the central randomisation group at Bayer HealthCare Pharmaceuticals using the software package SAS (Release 9.1 or higher; SAS Institute, Cary, NC, USA). Randomisation blocks of 12 were used. Eligible subjects were assigned their randomisation numbers via an interactive voice response system to avoid any assignment bias in this open-label study. With this system, neither the subject nor the investigator knew the identity of the study regimen before the assignment of the randomisation number.
Analysis set
The full analysis set (FAS) was defined as all women who received at least one dose of study medication and for whom at least one clinical observation following administration of study medication was available. The evaluation of the primary outcomes was based on the FAS, as was the evaluation of safety data. It should be noted that 67 subjects were excluded from the FAS because of invalid data owing to suspected misconduct at one study centre.
Sample size
In order to undertake a reasonable assessment of safety, it was considered that a treatment duration equivalent to 10 000 cycles of 28 days should be achieved for the flexibleMIB regimen. Based on previous experience with cycle control studies using conventional regimens, it was considered that at least 200 subjects per treatment arm would be sufficient to reliably describe the bleeding data.
Given that the flexibleMIB is the preferred regimen and assuming a difference of 10 days and a SD of 28 days plus an annual dropout rate of 40%, approximately 660 subjects were needed for the flexibleMIB regimen, and 225 subjects were needed for each of the other two regimens. This resulted in a proposed total sample size of 1110 subjects, which would result in a power of at least 94% to show superiority of the flexibleMIB regimen over the conventional regimen in terms of the primary bleeding variable.
The proposed sample size for the flexibleMIB group was also expected to provide sufficient statistical power to reliably determine contraceptive efficacy, as per the guidelines of the European Medicines Agency.
Results
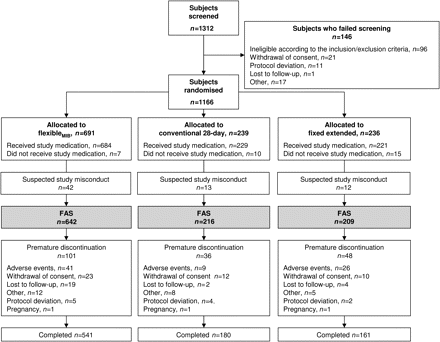
Overall, 1312 women were screened, and 1166 of these were subsequently randomised to one of the three regimens. Reasons for non-randomisation are shown in Figure 1. Study medication was administered to 1134 subjects in the three groups. After excluding the 67 subjects from a study centre with suspected study misconduct, 1067 women took at least one dose of study medication and had at least one clinical observation after administration of study medication; these women comprised the FAS (flexibleMIB, n=642; conventional, n=216; fixed extended, n=209; Figure 1). The demographic and baseline characteristics of women included in the FAS were generally similar in the three groups (Table 2). Study medication was completed by 88.5% of all subjects in the FAS. Overall, 83.9%, 80.3% and 72.9% of all women randomised to the flexibleMIB, conventional and fixed extended regimens, respectively, completed treatment. The mean treatment exposure times were 339 days for the flexibleMIB regimen, 340 days for the conventional regimen and 318 days for the fixed extended regimen.
Disposition of women in the 1-year comparative phase of the study. FAS, full analysis set (defined as subjects who received at least one dose of study medication and had at least one clinical observation after administration of study medication). MIB, management of intracyclic (breakthrough) bleeding.
Demographic and baseline characteristics of women who received ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set)
Primary outcomes
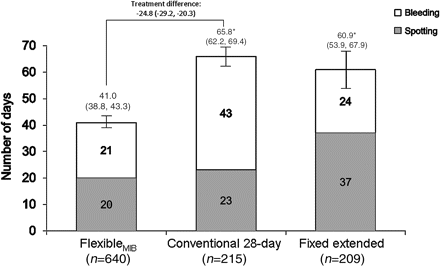
The mean number of bleeding/spotting days within the comparative phase was significantly lower with the flexibleMIB regimen than with the conventional regimen [41.0±29.1 (95% CI 38.8–43.3; n=640) vs 65.8±27.0 (95% CI 62.2–69.4; n=215) days, p<0.0001; treatment difference −24.8 (95% CI −29.2 to −20.3) days; Figure 2). The mean number of bleeding/spotting days with the fixed extended regimen was 60.9±51.1 (95% CI 53.9–67.9) days (n=209). No statistical testing was preplanned for this comparison; however, the between-treatment difference was in the same order of magnitude as that between the flexibleMIB regimen and the conventional regimen, and a post hoc analysis indicated that the flexibleMIB regimen significantly reduced the mean number of bleeding/spotting days versus the fixed extended regimen (p<0.0001). The variation in the overall number of days of bleeding in the first year in women who received the flexibleMIB regimen was similar to that in women who received the conventional regimen (Figure 3). The fixed extended regimen showed more variability (Figure 3).
Mean number of bleeding/spotting days in the comparative phase with ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set). Data are presented as mean (95% CI). The full analysis set was defined as all subjects who received at least one dose of study medication and had at least one clinical observation after administration of study medication. *p<0.0001 vs flexibleMIB (post hoc analysis for the fixed extended regimen comparison). MIB, management of intracyclic (breakthrough) bleeding.
Analysis of bleeding/spotting days in the comparative phase with ethinylestradiol 20 μg/drospirenone 3 mg administered as (a) flexibleMIB, (b) conventional 28-day and (c) fixed extended regimens (full analysis set). The full analysis set was defined as all subjects who received at least one dose of study medication and had at least one clinical observation after administration of study medication. MIB, management of intracyclic (breakthrough) bleeding.
Over the full 2 years of the study, the Pearl Index for the flexibleMIB regimen was 0.64 (95% CI 0.28–1.26) based on eight pregnancies and 1253 woman-years of exposure. One of these pregnancies was deemed to be a subject failure; the others were reported without information supporting subject failure, and were therefore classified as method failures. The cumulative pregnancy rate for up to 2 years of treatment (determined using the Kaplan–Meier life-table analysis) was 1.28% (95% CI 0.62–2.66). This corresponds to 99% contraceptive protection.
Secondary outcomes
Bleeding pattern
The flexibleMIB regimen was associated with the lowest mean number of bleeding/spotting days (analysed by 90-day reference periods). The mean number of bleeding/spotting days was generally higher with the conventional regimen at all time points (Table 3). During the 1-year comparative phase, the proportion of bleeding/spotting days was lowest in women treated with the flexibleMIB regimen (13.3±11.1%); corresponding proportions in recipients of the conventional and fixed extended regimens were 20.4±9.8% and 23.1±20.7%, respectively.
Bleeding pattern outcomes by 90-day reference period in women who received ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set)
Cycle control
The mean number of cycles in women who received the flexibleMIB, conventional and fixed extended regimens was 4.5±2.1 (median 4.0), 11.9±2.9 (median 13.0) and 2.8±0.9 (median 3.0), respectively. Values for mean cycle length were 78.2±39.8 (median 72.0), 27.9±1.8 (median 28.0) and 121.6±27.9 (median 124.0) days, respectively. The cycle length observed with the flexibleMIB regimen is shown in Figure 4. In women who received the flexibleMIB regimen, approximately 20% of all cycles were of the maximum length of 124 days.
Analysis of cycle length with ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB regimen (full analysis set). The full analysis set was defined as all subjects who received at least one dose of study medication and had at least one clinical observation after administration of study medication. MIB, management of intracyclic (breakthrough) bleeding.
Withdrawal bleeding episodes occurred approximately every 8–10 weeks in recipients of the flexibleMIB regimen and, as expected, every 4 weeks in recipients of the conventional regimen and every 124 days in recipients of the fixed extended regimen. The mean length of withdrawal bleeding episodes appeared to be shorter with the conventional 28-day regimen (4.4–5.2 days) than with the flexibleMIB regimen (7.5–14.2 days) and fixed extended regimen (2.0–10.5 days). For the majority of withdrawal bleeding episodes, the maximum intensity was reported as light to normal with all three regimens.
The mean number of intracyclic bleeding/spotting days during the comparative phase appeared similar in recipients of the flexibleMIB and conventional regimens, and lower than that in recipients of the fixed extended regimen (Table 4). A similar result was observed for the number of days with intracyclic bleeding only, and the mean number and maximum length of intracyclic bleeding episodes (Table 4). The maximum intensity of intracyclic bleeding was deemed spotting or bleeding with light intensity with all three regimens (Table 4).
Characteristics of intracyclic bleeding in women who received ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set)
The flexibleMIB regimen appeared to have a higher mean number of scheduled bleeding days (23.3±14.3 days) than the fixed extended regimen (14.0±5.9 days). In contrast, the opposite was observed for the number of unscheduled bleeding days, which was lower with the flexibleMIB regimen than with the fixed extended regimen (17.1±17.4 vs 46.6±48.4 days).
Subject questionnaires
Improvements in various items of the menstrual bleeding questionnaire were observed, compared with the 12 weeks prior to inclusion into the study. Between-treatment differences in symptoms were found, primarily as a result of extended cycles resulting in an absence of menstrual bleeding-related events. Aspects related to the PGWBI, user satisfaction and menstruation-related symptoms with the three regimens is shown in Table 5. Of those women who had painful menstruation before the start of the study, 52.5% treated with the flexibleMIB regimen and 58.1% treated with the fixed extended regimen reported feeling better having received treatment. In addition, 86.0% and 79.1% of women, respectively, would recommend their regimen to a friend.
User satisfaction, Psychological General Well-Being Index score and menstruation-related symptoms in women who received ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set)
Safety
The occurrence of AEs during the comparative phase is reported in Table 6. The vast majority (93.1%) of AEs that occurred were mild or moderate in intensity; there was no difference in AE intensity between the three groups.
Adverse events occurring in the comparative phase of the study with ethinylestradiol 20 μg/drospirenone 3 mg administered as a flexibleMIB, conventional 28-day or fixed extended regimen (full analysis set)
There were four serious AEs (SAEs) that were considered to be possibly related to study medication: focal nodular hyperplasia of the liver, uterine leiomyoma and two cases of deep vein thrombosis (DVT). More detailed data pertaining to long-term safety outcomes during the 2-year study are reported separately.18
Discussion
The results of this trial show that EE 20 μg/DRSP 3 mg administered in a flexibleMIB regimen was associated with significantly fewer bleeding/spotting days over a period of 1 year compared with the conventional regimen. It was also associated with a similarly significant reduction in the number of days of bleeding/spotting than the fixed extended regimen. In addition, the flexibleMIB regimen showed good contraceptive efficacy similar to other COCs and was well tolerated.
The flexibleMIB regimen had a more favourable effect on various aspects of menstrual bleeding than the two other regimens, including fewer bleeding/spotting days overall and fewer days with unscheduled bleeding. The reduction in bleeding/spotting days with the flexibleMIB regimen compared with the conventional regimen was mainly related to 50% fewer days of menstruation-like bleeding. The reduction in bleeding/spotting days with the flexibleMIB regimen compared with fixed extended regimen could be related to one-half the number of days with spotting only (Figure 2). Overall, the flexibleMIB regimen was associated with a reduced proportion of bleeding days of any intensity; by one-third compared with the conventional regimen and potentially by one-third compared with the fixed extended regimen. In addition, the flexibleMIB regimen appeared to be associated with less variation in the overall number of days of bleeding/spotting than the fixed extended regimen. On average, women taking the flexibleMIB regimen can expect to have approximately 40 days of bleeding per year in comparison to approximately 65 days with the conventional regimen (corresponding to 5 days of bleeding per cycle).
The mean number of intracyclic bleeding/spotting days in recipients of the flexibleMIB regimen seemed to be markedly lower than that in recipients of the fixed extended regimen. This may be because women who received the latter regimen were instructed to take 120 days of uninterrupted active hormonal intake irrespective of any bleeding/spotting. In contrast, women who received the flexibleMIB regimen who experienced three consecutive days of bleeding/spotting during Days 25–120 were instructed to observe a 4-day tablet-free interval; in this manner, the 3 days of bleeding/spotting that preceded the tablet-free interval became part of the bleeding episode of the withdrawal bleed. As such, the length of the whole bleeding episode in the flexibleMIB group was prolonged by 3 days because of the stipulated ‘wait and see’ period of three consecutive days of bleeding/spotting. Ultimately, this may translate into fewer bleeding days overall and better cycle control with fewer episodes of intracyclic bleeding, which could improve the acceptability of the flexibleMIB regimen compared with the fixed extended regimen.
The acceptability of the flexibleMIB regimen may also be influenced by the fact that the onset of bleeding could be managed, within the limitations of the regimen. With the flexibleMIB regimen women could take treatment for 24 days, 120 days, or for an amount of time between 25 and 120 days, depending on the occurrence of bleeding/spotting. Although this study did not allow the flexibleMIB regimen group to observe a 4-day tablet-free interval at any time between Days 25 and 120 (i.e. independent of the occurrence of bleeding/spotting), in clinical practice women may do so. In fact, in the flexibleMIB group 5.9% of all cycles ended with a 4-day break during the flexible intake phase without the preceding days of intracyclic (breakthrough) bleeding. The importance to women of being able to manage their withdrawal bleeding should not be underestimated. Many women find menstrual bleeding both painful and inconvenient,7 with 75.6%, 28.8% and 48.4% of women reporting that their menstrual bleeding interfered with their sex lives, work lives or sporting activities, respectively.19 The freedom to decide when their menstrual bleeding occurs also appears to be important to women; data indicate that women using COCs will deviate from the recommended intake pattern in order to prevent bleeding during special occasions or holidays/weekends.3 In the current study, approximately 20% of all cycles were of the maximum length of 124 days, indicating that around 80% of the intake cycles required flexibility in length in order to manage three consecutive days of bleeding/spotting.
In this study, the tolerability of the three regimens was comparable based on an assessment of AEs occurring during the first year of use and similar to those reported for other low-dose COCs, including EE 20 μg/DRSP 3 mg in a 24/4 regimen.15,–,17 20,–,22 It should be noted that the type of AE reporting did not allow for an assessment of the frequency of AEs in an individual woman. A similar AE rate between the three groups, therefore, does not necessarily mean a similar frequency in individual women. As such, the expectation that menstrual-related complaints will occur less frequently when a woman has less frequent menstrual periods is not disproved by this finding. Four SAEs that were considered to be possibly related to study medication were recorded: focal nodular hyperplasia, uterine leiomyoma and two cases of DVT. A more comprehensive overview of the safety and tolerability of the three regimens investigated in the current study is reported elsewhere.18
Comparison of the bleeding profile of the flexible extended regimen in this study with fixed extended COC regimens is difficult, primarily because of the way in which treatment was administered and data were collected. Specifically, beyond the first 24 days of mandatory intake, cycle length with the flexible extended regimen was variable because women had to take a 4-day tablet-free interval between Days 25 and 120 if they experienced three consecutive days of bleeding/spotting; in contrast, in studies of fixed extended regimens, cycle length was fixed. Some previous studies have, however, reported the overall number of bleeding/spotting days. In a 1-year study, women were randomised to receive EE 30 µg/levonorgestrel (LNG) 150 µg in a 91-day extended regimen for four cycles (n=456) or EE 30 µg/LNG 150 µg in a 21/7 regimen for 13 cycles (n=226).12 There was a reduced number of bleeding/spotting days in women who received the fixed extended regimen (median 35 days) versus the conventional 28-day regimen (median 53 days), similar to the findings reported here. The proportion of women on the EE/LNG fixed extended regimen who prematurely discontinued medication because of any AE or bleeding irregularities was 14.9% and 7.7%, respectively. In the current study these values were lower: 6.4% and 1.2% with the flexibleMIB regimen, respectively. In a second study, women received EE 20 μg/LNG 100 μg (n=16) administered for 168 days without a pill-free interval or as six 28-day cycles (n=16) on an open-label basis.13 Over the course of the study, women on the fixed extended regimen had a mean of 25.9±29.2 days of bleeding/spotting. In a similarly designed, double-blind study, in which 31 women received EE 20 μg/norethindrone acetate 1 mg for 168 days, 31.5±21.8 days of vaginal bleeding were reported.14 It should be noted that the latter two studies were conducted over 168 days, whereas bleeding outcomes in the current study are reported for a full year.
Conclusions
The results of this study show that EE/DRSP administered as a flexibleMIB regimen is associated with fewer days of bleeding/spotting and fewer withdrawal bleeding episodes over 1 year compared with the same hormonal combination in a conventional 28-day regimen. It also appeared to provide fewer spotting days compared with the same combination in a fixed extended 124-day regimen. The flexibleMIB regimen provides reliable contraceptive efficacy, and has a comparable tolerability profile to the conventional and fixed extended regimens. The flexibleMIB regimen is unique in that it extends the menstrual cycle and provides women with the flexibility to manage their intracyclic (breakthrough) bleeding.
Acknowledgments
The authors would like to thank Lyndal Staples and Clare Wheatcroft of inScience Communications for medical writing support and Bodo Kirsch of Bayer HealthCare Pharmaceuticals for statistical support. This work was funded by Bayer HealthCare Pharmaceuticals, Berlin, Germany. The authors would like to thank Martina Bongartz of Bayer HealthCare Pharmaceuticals for preparation of the clinical study report.
References
Footnotes
-
Funding This study was funded by Bayer HealthCare Pharmaceuticals, Berlin, Germany, the manufacturer of of ethinylestradiol 20 μg/drospirenone 3 mg.
-
Competing interests Christine Klipping and Ingrid Duijkers are employees of Dinox BV, one of the centres in which the study was conducted. Michael P Fortier is a physician collaborating with the Clinique de Recherche en Santé des Femmes, one of the centres in which the study was conducted. Joachim Marr, Dietmar Trummer and Jörg Elliesen are employees of Bayer HealthCare Pharmaceuticals.
-
Provenance and peer review Not commissioned; externally peer reviewed.